What you need to know to protect yourself and your bees.
Authors: Philip Moore, Michael Wilson, John Skinner
Department of Entomology and Plant Pathology, the University of Tennessee, Knoxville TN
Date: January 8, 2015
Introduction
The African honey bee (Apis mellifera scutellata) was introduced from the savannahs of eastern and southern Africa to the eucalyptus forests of São Paulo Brazil by Professor Warwick E. Kerr in 1956. Kerr described the colonies as “the most prolific, productive, and industrious bees.” His intention was to breed a race of honey bee that would be more adapted to the tropical climate of South America than the temperate climate adapted European races of honey bee. Although African bees were originally considered to be “accidentally released”, several studies including those published by Kerr (1967), do not uphold that view. Kerr performed hybridization experiments, reared and artificially inseminated African queens with Italian honey bee (A. m. lingustic) drones, and distributed these bees to beekeepers in southern Brazil (Kerr 1967). Although the spread of the African honey bee was quickly recognized in Brazil and Argentina, the first English language published accounts of problems with defensiveness of the African bees came in 1964 by Nogueira-Neto. By 1972, the US had taken notice and published a report of the impending problem.
In 1990 the much anticipated arrival of the original African bees’ descendants, now called Africanized honey bees or “killer bees” reached the southern US and in 2 years, had spread to Arizona, New Mexico, and southwestern Texas. Africanized honey bees (AHB) are now found throughout much of South, Central and southern North America, but may have reached an upper and lower limit to their spread at about the 34°N and 34°S latitude (Visscher et al 1997; Kerr et al 1982), based in part to their inability to survive extended cold periods (Taylor 2003; Villa et al 1991, 1993). As Africanized honey bees spread, more than 1000 people and tens-of-thousands of domestic animals were killed in stinging incidents (Breed et al 2004). This created a fear-driven public health crisis and forced many beekeepers in South America out of business, but we now have a better understanding of Africanized honey bees’ defensive behavior and beekeeper’s coping mechanisms. Beekeeping in South and Central America has since rebounded and commercial US beekeepers have had few issues.
Description
The key to understanding the differences in Africanized honey bees and European honey bees is to recognize that they are the same species and therefore very similar, except for having evolved to persist in significantly different habitats. No honey bees (Apis mellifera) are native to the New World. Nearly all of the honey bees used for beekeeping in North America, and up until recently South America, were imported from Europe and were adapted to survive in temperate climates. These include many subspecies like Italians (A. m. linguistic), Carniolans from Slovenia and the Balkans (A. m. carnica), and the European black bee, also known as the German black bee (A. m. mellifera). These European bees are good honey producers in the temperate climates of North America. They evolved to construct large, honey laden nests in order to survive the winter. African honey bees (A. m. scutellata), in contrast, evolved to survive in tropical climates, and thus do not have winter to contend with, but instead are limited by rainfall and floral availability. Therefore, they build smaller nests, do not store large amounts of honey, are sensitive to cold, and migrate to find a new home when floral resources diminish. However, Africanized bees are hybrids of European and A. m. scutellata genetics and the expression of these behaviors may vary in Africanized bees. Distinguishing between these subspecies is possible through laboratory diagnostics by morphometrics or genetic analysis. To the average person, the greatest difference between the European and African honey bees is their defensive behavior.

Better understanding: Africanized bee (left) and European bee (right) are indistinguishable to the un-aided eye. The color difference seen here can also be found in European honey bees. Credit: Scott Bauer. Courtesy: USDA-ARS
Defensive Behavior
Defensive response of Africanized honey bees is more rapid, intense, and involves a greater proportion of the colony population. A study of honey bees in Africa suggests that African bees (A. m. scutellata) have a lower threshold for defensive response and react more intensely, faster, and in larger numbers (Schneider and McNally 1992). When given the same stimuli, Africanized bees in the New World sting 4 to 10 times more frequently (Guzman-Novoa et al 1999, 2002a, 2002b; Guzman-Novoa and Page 1993, 1994) and pursue with 10 to 30 times more bees than European colonies (Guzman-Novoa et al 2003, Prieto-Merlos 2002; Stort and Goncalves 1991). One reason for the Africanized bees more intense response may be greater alarm pheromone release; one study found 9 of 12 alarm chemical components were stronger in Africanized bees than European bees (Collins et al 1989). Defensive bees may also be more sensitive to alarm pheromones (Harris and Woodring 1999).
Another aspect of nest defense, the number and duration of workers guarding the entrance, may be reinforced by the alarm pheromone of Africanized bees (Hunt et al 2003). Guarding is a distinct worker defensive task, whereas soldiers are any bees involved in pursuing and stinging intruders. Guards compose about 10 percent of the colony population. They are usually middle aged (between 13 and 16 days old) and will remain a guard for one to three and up to six days (Hunt et al 2003). In one study, European guards persisted in that role for 3 days, where Africanized bees guarded for 4.7 days on average (Hunt et al 2003). The longer a bee remains a guard, the more responsive it becomes to alarm pheromones (Breed and Rogers 1991, Breed et al 1989).
The primary purpose of guard bees is to defend against robbing, identifying and removing conspecific intruders (Breed 1991, Breed et al 1992), but guards may also influence recruitment of other soldier bees to defend against larger intruders. Few guard bees of European colonies fly out and orient towards an intruder, instead they produce alarm pheromone to recruit soldiers to attack. Africanized guards are seven times more likely to fly out and sting (Guzman et al 2003). In a maximal defensive act, an estimated 10 percent of European workers in a colony may be involved, whereas 50 percent of Africanized workers may pursue and sting under certain circumstances (Breed 1991); even foragers with pollen loads have been observed stinging (Prieto-Merlos 2002). The defensive perimeter for Africanized bees is also greater and soldiers will attack intruders 100 or more meters from the nest, pursing intruders for several kilometers, while European honey bees will only defend about a 50 meter perimeter (Spivak et al 1991).
Defensive behavior is influenced by genetic heritability, exemplified by different races of honey bees with different defensive characteristics (Ruttner 1988). However, since a worker population may contain genes from up to 17 different drone fathers, the defensive behavior of a honey bee colony is highly variable. Even in colonies with a low proportion of aggressive bees, the defensive bees in the minority may recruit other less aggressive bees into stinging (Guzman-Novoa et al 2003). Highly defensive behavior, measured by number of stings, may be a dominant trait at the colony level (DeGrandi-Hoffman et al 1998; Guzman-Novoa and Page 1993, 1994). Other environmental factors and stimuli also influence defensive behavior.

Better prepared; Eric Erickson trains first responder John Estes of Tucson Arizona to use soapy water to neutralize an Africanized bee attack. Credit: Jack Dykinga. Courtesy of: USDA-ARS
Invasion
The colonization of much of the western hemisphere by the African honey bee (Apis mellifera scutellata) in the last 60 years is one of the most rapid and impressive biological invasions in recent history (Schneider et al 2004). Originally, African honey bees were thought to displace European honey bee subspecies through hybridization and to give rise to “Africanized honey bees” in Latin America. However the incredible success of African bees that have invaded European populations, has led to much of the European characteristics to be lost and existing honey bee populations to remain essentially African in their nesting behavior (McNally and Schneider 1992), swarming and absconding behavior (Otis et al 2002; Rubink et al 1996; Schneider 1995; Schneider and McNally 1992 and 1994; Sousa et al 2002), foraging and diet selection (Fewell and Bertran 2002; Schneider and Hall 1997; Schneider and McNally 1993), and maternal DNA characteristics (Clarke et al 2001 and 2002; Hall 1999; Segura 1989).
The effect of African populations on feral colonies in the Neotropics (including all of South and Central America along with the Caribbean, Southern Florida and Mexico) is especially acute in terms of genetic introgression. When African bees first expand into a new location, both European and African genetic markers are found, but after 5-10 years, African genes predominate and European genes typically decrease to less than 10 percent (Schneider et al 2003). At least six different mechanisms are responsible for the loss of European genetic lineage. The relative effect of these mechanisms differs depending on managed or feral settings. For example, because of the effect of re-queening in commercial apiaries, European maternal lineage is more likely to be retained, while open mating contributes to paternal African introgression. The paternal affect of Africanized introgression is likely to bring increased defensive behavior into stocks quickly as defensive behavior seems to be significantly influenced by paternity (Guzman-Nova et al 2005).
Growth Rate and Swarming
African colonies have a greater emphasis on pollen collection than European colonies (Fewell and Bertram 2002; Franck et al 2000) and convert the pollen into greater populations of brood (Schneider and McNally 1994; Spivak et al 1991), devoting as much as two to four times more comb area to brood rearing (McNally and Schneider 1992 and 1996). The higher growth rate allows African bees in the Neotropics to swarm up to 16 times per year, compared to 3 to 6 times for feral European colonies (Otis 1991; Winston 1992). The increased swarming allows African colonies to increase in density quickly and lead to overrepresentation of African genetics in the available mating pool.
Hybrid Bees
One factor that may be contributing to the loss the European genes is the reduced fitness of hybrid bees resulting from incompatibilities between European maternal and African paternal alleles. European bees have adapted to temperate climates, where African bees are adapted to tropical environments, which may reflect some of these incompatibilities. Hybrid workers exhibit greater frequency in small differences in wing symmetry resulting from developmental disturbances (Schneider et al 2003). Hybrids also have lower metabolic rates when reared from European maternity (Harrison and Hall 1993). These disruptions may affect flight performance and colony dispersal ability. Hybrids may also be less efficient foragers (Quezada-Euán and Hinsull 1996), store less honey, and produce less brood (Taylor 1999 and 2003). Although the negative effect of hybridization remains a controversial aspect of the African bee invasion (Rinderer et al. 1991 and 1993; Sheppard et al 1991; Lobo 1995; Quezada-Euán and Hinsull 1995), it does exemplify potential consequences in the long-term survival of European lineage in overlapping habitat.
Drone Advantage in Mating
Several advantages allow African drones to disproportionately mate with European queens. One primary component is the sheer numerical advantage. African colonies produce a greater proportion of drones than European colonies in the same setting (Rinderer et al 1987; Otis et al 2002). This is due in part to a greater swarming tendency; however a couple of other interesting attributes also contribute. African drones will invade and habitat in European colonies, which may suppress the production of European drones (Rinderer et al 1985) and higher rates of queen loss in African colonies leads to queen-less colonies that rear large numbers of worker-produced drones (DeGrandi-Hoffman and Schneider 2002; Zillikens et al 1998). Other than greater abundance, other factors that may contribute to African drone mating success are not well understood, such as higher rates of sperm usage by queens mated with African and European drones (DeGrandi-Hoffman et al 2003).
Paternal African Virgin Queens
When virgin queens produced by European colonies mate with African and European drones, the resulting colony will be composed of paternal African and European workers. In the next queen replacement cycle, these colonies will rear virgin queens from both African and European paternal lineage, but those from African paternity have a competitive advantage. Paternal African virgin queens develop faster and therefore emerge earlier than their European paternity counterparts, which may give them the opportunity to eliminate rivals confined in their queen cells (DeGrandi-Hoffman et al 1993 and 1998; Schneider and DeGrandi-Hoffman 2002 and 2003). Paternal African queens also kill more rivals than their European-paternity sister queens, produce more “piping” sounds that may prevent emergence of virgin queens or enhance dueling success, and attract workers to perform more “vibration signals” that may promote queen survival (Schneider and DeGrandi-Hoffman 2003; Schneider et al 2001). These factors in combination may result in paternal African queens becoming more likely to become the new laying queen of these colonies. With each new queen replacement cycle, virgin queens disproportionately mate with African drones, and African genetic introgression into European colonies continues.
African Allelic Dominance
In Neotropical areas where a high proportion of European genetics persist, honey bee colonies often exhibit African behavioral traits; therefore African alleles may be dominant for some characteristics, further contributing to the spread of the African pheonotype. African alleles may be dominate for worker foraging (Fewell and Bertram 2002; Schneider and Hall 1997), queen behavior (Schneider and DeGrandi-Hoffman 2003), and resistance to the Varroa mite (Guzmán-Novoa et al 1996). However the most notable behavior is the aggressive defensive behavior shown by African bees. This aggressive behavior appears to be linked to paternal rather than maternal factors. Colonies of hybrid workers composed of a European queen mated with New World Africanized drones show defensiveness not different from African bees (DeGrandi-Hoffman et al 1998; Guzmán-Novoa and Page 1994; Guzmán-Novoa et al 2002; Hunt and Guzmán-Novoa 2002), while colonies headed by an African queen mated with European drones show decreased defensiveness (DeGrandi-Hoffman et al 1998), although still greater than European colonies. Since queens mate with up to 17 drones, the aggressiveness of the colony may be dependent on the relative abundance of paternal African workers in the population.
Nest Usurpation
Nest usurpation is a form of parasitism where African swarms invade European colonies, replacing the queen and therefore eliminate the European maternal lineage. The frequency of usurpation is regionally variable; between 0 and 40 percent of colonies annually has been reported in different regions of Mexico (Vergara et al 1993), 5 percent in Venezuela (Danka et al 1992), and 25 percent in southern Arizona (Schneider et al 2004). Unfortunately, nest usurpation is among the least understood aspects of the African bee invasion and it is unknown how African swarms find and invade a host colony. It is likely that unknown pheromones are involved in associating the condition of the colony; particularly susceptible colonies are queenless or have a caged queen, however queenright colonies can also be invaded (Dietz et al 1989).

Better understanding: Judith Hooper and David Gilley analyze volatiles from Africanized bee pheromones involved in nest usurpation. Credit: Scott Bauer. Courtesy of: USDA-ARS
The Africanized Honey Bee in the United States
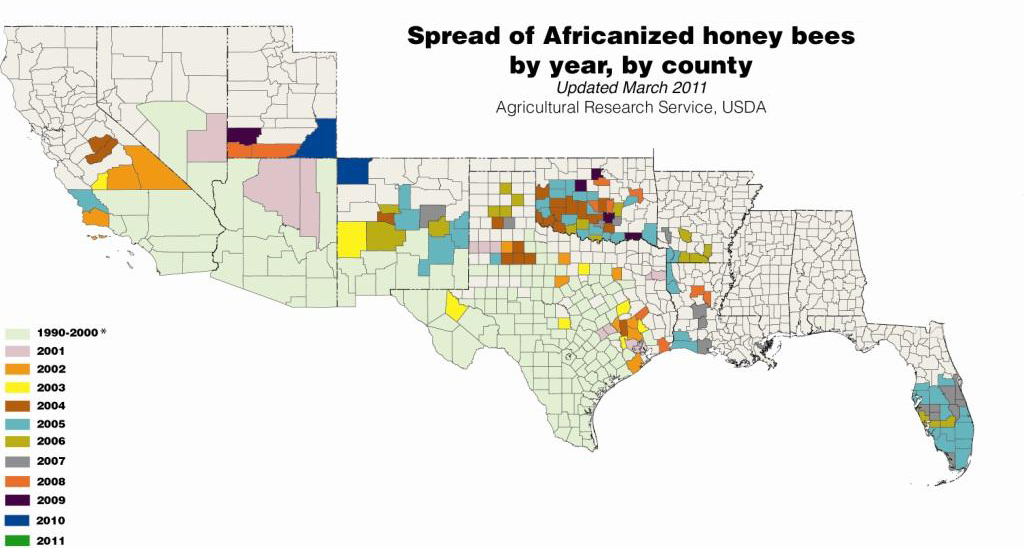
The African bee first appeared in Texas in 1990 (Hunter et al 1993) and has since spread throughout Texas, and Arizona, through most of New Mexico and Oklahoma, and parts of Arkansas, Louisiana, Florida, California, Nevada, and Utah. Africanized bees are displacing European genotypes as has been seen in Latin America (Loper 2002, Looper at al 1999; Schneider et al. 2004), however the spread is slower and more erratic. The slower spread may be due to limited winter survivability (Taylor 2003; Villa et al 1991 and 1993); and preference for arid conditions (Villa et al 2002; Ruttner 1998), which may confine the spread to the southern regions of the US. In addition, the adaptations that allow African bees to adapt to tropical climates may inhibit their expansion to temperate climates, which have vastly different pollen and nectar resource availability and day length (Schneider and McNally 1992; Rinderer 1993; Villa et al 1993). The affect of migratory beekeeping practices, tracheal and Varroa mites, and density of European colonies on the spread of African bees in the US is not well understood.
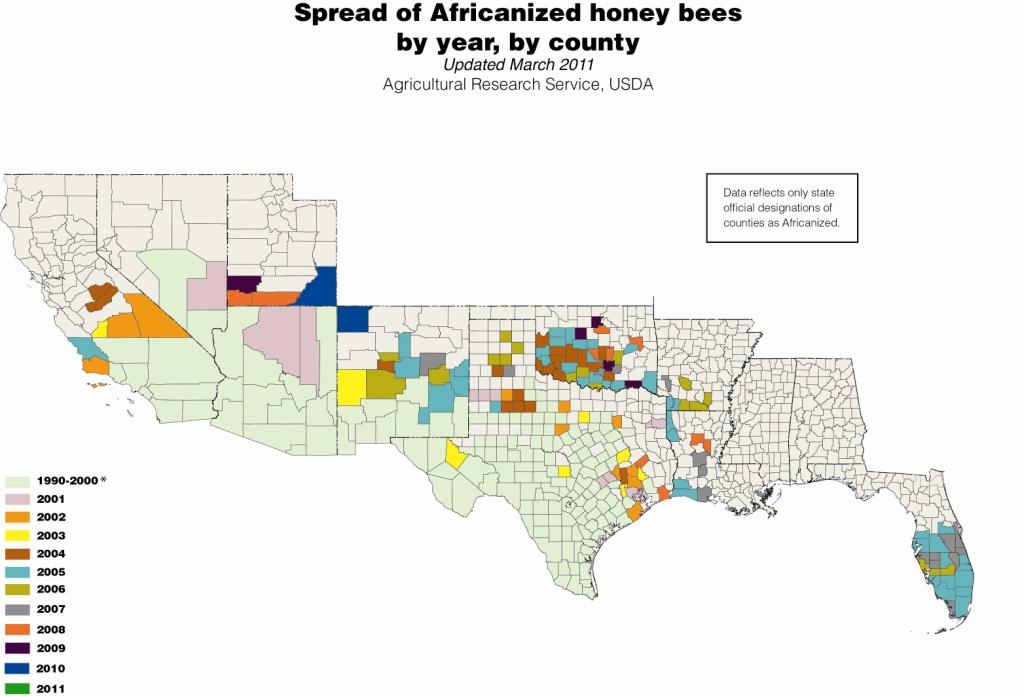
Better understanding: Distribution and spread of Africanized bees in the US 1990-2011. Courtesy of USDA-ARS
The economic affect of Africanized bees on US agriculture has been less severe than anticipated, perhaps due to the slower than expected spread and lessons learned from experiences in Latin America. The use of African bees in US agricultural practices is considered unworkable. However, African bees have been successfully integrated into many Latin American agricultural practices (Ratniek and Visscher 1996; Guzman-Novoa and Page 1994). Some beneficial characteristics of African bees like reduced susceptibility to mites, some bacterial diseases, and pesticides may be valuable (Arechavaleta-Velasco and Guzman-Novoa 2002; Danka et al 1986; Danka and Villa 1996; Ratnieks and Visscher 1996). However, utilizing African bees requires substantial alteration in beekeeping techniques and requeening with European queens is still necessary.
Africanized Bees and the Varroa Mite
Varroa mites (Varroa destructor) are considered the most serious threat to European honey bee colonies (De Jong et al 1982). However the interaction of Africanized bees with Varroa mites is strikingly different from that of European bees. Although the relationship varies by region, honey bees in much of South America appear to not be impacted by Varroa parasitism (Calderon et al 2010). One aspect of resistance is grooming behavior. Moretto et al (1991) found grooming behavior of African bees in Brazil to be eight times more efficient at removing Varroa than Italian bees and 31 percent of infested African honey bee workers removed Varroa by their own or another bee’s grooming action (Moretto 1997). These results were confirmed by Arechavaleta-Velasco and Guzman-Novoa (2001) in Mexico, however, Vandame et al (2002) only found eleven percent of mites removed by African bees compared to eight percent by European bees in Mexico. Therefore alternative attributes are possibly conferring resistance. Workers of Africanized bee colonies are more efficient at removing Varroa infested brood than European workers in the same conditions, displaying superior hygienic behavior (Moretto et al 1991; Spivak and Gilliam 1998; Vandame et al. 2000 and 2002). Shorter development time of pupating African bees (10-13 hours less than European bees) is also suggested to limit the reproduction of mites (Camazine 1986; Ritter and De Jong 1984). All of these factors are expected to reduce the reproductive potential of female Varroa mites.
Practical Implications
Living with Africanized bees, where they exist, requires caution and diligence, but not alarm. Sensationalist news accounts of “killer bees” have mostly been over-blown, but the risk to unsuspecting or unwitting people and animals is still possible. Africanized bees will only sting to defend their nest. If you come upon a bee nest, leave the area immediately and contact the local extension office or a pest-control company. Protecting oneself during a bee stinging event requires seeking immediate shelter in a building or car. Run as quickly as possible to shelter. Do not enter a body of water; the bees will wait for you at the surface. Do not swat at the bees or attempt any other defense; escaping the scene is paramount.
Once you have reached shelter, treat the stings like a typical honey bee sting. Remove the stinger by scraping with a fingernail or credit card as soon as possible and apply ice to reduce swelling and pain. Any abnormal reaction like shortness of breath, hives, or lightheadedness will require a trip to the emergency room for epinephrine. In minor cases the pain and swelling will subside in a few hours.
Animals should not be tied up, preventing their escape. Confined animals are the most common victims of Africanized bees. Areas around the home should be free of debris that allows a swarm to colonize. Any opening greater than 1/8 inch may provide a cavity and should be sealed. Swarming activity peaks in May-June, use caution and inspect your property around this time. Look for bees coming and going to identify a possible nest location. Do not attempt to investigate or move an object if activity is seen, contact a professional and wear protective clothing before entering the area.

Better understanding, better prepared: Mona Chambers, measuring an Africanized honey bee wing, one of the traits that differentiate these bees from European bees. Credit: Scott Bauer. Courtesy of: USDA-ARS
Information for first-responders can be found here. Beekeepers with aggressive colonies that are suspected of being Africanized should contact their state Apiary Inspector. Requeening may suffice to reduce the aggression; otherwise the colony may need to be destroyed to prevent its spread and potential liability. Identifying honey bees as Africanized is accomplished through morphometrics. To submit a sample for diagnosis see here.
References
Arechavaleta-Velasco, M. E. and E. Guzmán-Novoa. 2001. Relative effect of four characteristics that restrain the population growth of the mite Varroa destructor in honey bee (Apis mellifera) colonies. Apidologie, 32(2): 157-174.
Arechavaleta-Velasco, M.E. and E. Guzman-Novoa. 2002. Relative contribution of four mechanisms to the resistance of honey bees Apis mellifera L. against the mite Varroa jacobsoni Oud. In Erickson, E. R.E. Page, A.A. Hanna (eds) Proceedings of the 2nd International Conference on Africanized Honey Bees and Bee Mites. Root: Medina, OH.
Breed, M.D. 1991. Defensive behavior. In Spivak, M., D.J.C. Flecher, M.D. Breed (eds.) The “African Honey Bee. Westview. Boulder CO.
Breed, M.D. and K.B. Rogers. 1991. The behavioral genetics of colony defense in honeybees: genetic variability for guarding behavior. Behavi. Genet. 21: 295-03
Breed, M.D., K.B. Rogers, J.A. Hunley, and A.J. Moore. A correlation between guard behavior and defensive response in the honey bee, Apis mellifera. Anim. Behav. 37: 515-16.
Breed, M.D., T.A. Smith, A. Torres. 1992. Role of guard honey bees (Hymenoptera: Apidae) in nestmate discrimination and replacement of removed guards. Ann. Entomol. Soc. Am. 85:633-37.
Breed, M. D., E. Guzman-Novoa, G. J. Hunt. 2004. Defensive behavior of honey bees: organization, genetics, and comparisons with other bees. Annu. Rev. of Entomol. 49: 271-98
Calderón, R. A., J. W. Van Veen, M. J. Sommeijer and L. A. Sanchez. 2010. Reproductive biology of Varroa destructor in Africanized honey bees (Apis mellifera). Experimental and Applied Acarology, 50(4): 281-297
Camazine, S. 1986. Differential reproduction of the mite, Varroa jacobsoni (Mesostigmata: Varroidae), on Africanized and European honey bees (Hymenoptera: Apidae). Annals of the Entomological Society of America, 79(5): 801-803
Clark, K.E. T.E. Rinderer, P. Franck, J.G. Quezada-Euan, B.P. Oldroyd. 2002. The Africanization of honey bees (Apis mellifera L.) of the Yucatan: a study of a massive hybridization event across time. Evolution 56:1462-74.
Clarke, K.E., B.P. Oldroyd, G. Quezada-Euan, T.E. Rinderer. 2001. Origin of honey bees (Apis mellifera L.) from the Yucatan peninsula inferred from mitochondrial DNA analysis. Mol. Ecol. 10: 1347-55
Collins, A.M., T. E. Rinderer, H.V. Daly, J.R. Harbo, D. Pesante. 1989. Alarm pheromone production by two honeybee (Apis mellifera) types. J. Chem. Ecol. 15:1747-56
Danka, R. G., and J.D. Villa. 1996. Comparative susceptibility of Africanized honey bees from South Texas to infestation by Acarapis woodi. Southwestern Entomologist, 21, 451-456.
Danka, R.G., T.E. Rinderer, R.L. Hellmich, A.M. Collins. 1986. Comparative toxicities of four tropically applied insecticides to Africanized and European honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 79:18-21
Danka, R.G., R.L. Hellmich, T.E. Rinderer. 1992. Nest usurpation, supersedure and colony failure contribute to Africanization of commercially managed European honey bees in Venezuela. J. Apic. Res. 31:119-23
De Jong, D., R.A. Morse, and G.C. Eickwort. 1982. Mite pests of honey bees. Annual Review of Entomology, 27(1), 229-252.
DeGrandi-Hoffman, G. and S.S. 2002. Worker behaviors in queenless Africanized honey bee colonies. In Erickson, E. R.E. Page, A.A. Hanna (eds) Proceedings of the 2nd International Conference on Africanized Honey Bees and Bee Mites.
DeGrandi-Hoffman, G., M. Spivak, J.H. Martin. 1993. Role of thermoregulation by nestmates on the development time of honey bee (Hymenoptera: Apidae) queens. Ann. Entomol. Soc. Am. 86: 165-72
De Grandi-Hoffman, G., A.M. Collins, J.H. Martin, J.O. Schmidt, H.G. Spangler. 1998. Nest defense behavior in colonies from crosses between Africanized and Eurpean honey bees (Apis mellifera L.) (Hymenoptera:Apidae). J. Insect Behavior. 11:37-45
DeGrandi-Hoffman, G., D.R. Tarpy, S.S. Schneider. 2003. Patriline composition of worker populations in honey bee (Apis mellifera L.) colonies headed by queens inseminated with semen from African and European drones. Apidologie 34: 111-20
DeGrandi-Hoffman, G., A. Collins, J.H. Martin, J.O. Schmidt, H.G. Spangler. 1998. Nest defense behavior in colonies from crosses between Africanized and European honey bees (Apis mellifera L.) J. Insect Behav. 11:37-45
Dietz, A., C. Vergara, M. Mejia, R. Krell. 1989. Forced queen usurpation in colonies of Africanized and European honey bees in Argentina. Proc. XXXVII Apimondia Int. Congr., Rio de Janeiro, Brazil, pp 88-92. Bucharest, Rom.: Apimondia Publ.
Fewell, J.H. and S.M. Bertram. 2002. Evidence for genetic variation in worker task performance by African and European honey bees. Behav. Ecol. Sociobiol. 52: 318-25
Franck, P., L. Garnery, G. Celebrano, M. Solignac, J-M. Cornuet. 2000. Hybrid origins of honey bees from Italy (Apis mellifera ligustica) and Sicily (A. m. sicula). Mol. Ecol. 9: 907-21
Guzman-Novoa, E, and R.E. Page. 1993. Backcrossing Africanized Honey bee queens to European drones reduces colony defensive behavior. Ann. Entomol. Soc. Am. 86: 352-55
Guzman-Novoa, E, and R.E. Page. 1994. Genetic dominance and worker interactions affect honey bee colony defense. Behav. Ecol. 5: 91-97
Guzmán-Novoa, E., R.E. Page. 1994. The impact of Africanized bees on Mexican beekeeping. Am. Bee J. 134: 101-6
Guzmán-Novoa, E. A. Sanches, R.E. Page, T. Garcia. 1996. Susceptibility of European and Africanized honey bees (Apis mellifera L.) and their hybrids to Varroa jacobsoni Oud. Apidologie 27: 93-103
Guzmán-Novoa, E., G.J. Hunt, R.E. Page, M.K. Fondrk 2002a. Genetic correlations among honey bee (Hymenoptera: Apidae) behavior characteristics and wing length. Ann. Entomol. Soc. Am. 95: 402-6
Guzman-Novoa, E., R.E Page, H.G. Spangler, E.H. Erickson. 1999. A comparison of two assays to test the defensive behavior of honey bees (Apis mellifera). J. Apic. Res. 38: 502-9
Guzmán-Novoa, E., G.J. Hunt, J.L . Uribe, C. Smith, M.E. Arechavaleta-Velasco. 2002b. Confirmation of QTL effects and evidence of genetic dominance of honey bee defensive behavior: results of colony and individual behavior assays. Behav. Genet. 32:95-102
Guzmán-Novoa, E., G.J. Hunt, J.L . Uribe, D. Prieto-Merlos. 2004. Genotypic effects of honey bee (Apis mellifera) defensive behavior at the individual and colony levels: the relationship of guarding, pursuing and stinging. Apidologie, 35(1), 15-24.
Guzman- Novoa, E.,G. J. Hunt, R. E. Page, J.L. Uribe-Rubio, D. Prieto-Merlos, and F. Becerra-Guzman. 2005. Paternal effects on the defensive behavior of honeybees. Journal of Heredity, 96(4), 376-380.
Hall, H.G., 1999. Genetic and physiological studies of African and Wuropean honey bee hybridizations: past, present and into the 21st century. In Hoopingardner, R., and L. Conner (eds). Apiculture for the 21st Century. Wicwas: Cheshire CT.
Harris, J.W. and J. Woodring. 1999. Effects of dietary precursors to biogenic amines on the behavior of honey bees (Apis mellifera) to the alarm pheromone component isopentyl acetate. Physiol. Entomol. 24:285-91
Harrison, J.F. and H.G. Hall. 1993. African European honey bee hybrids have low non-intermediate metabolic capacities. Nature 363: 258-60
Hunt, G.J. and E. Guzmán-Novoa 2002. Behavior genetics of defensive behavior in Africanized honey bees. In Erickson, E. R.E. Page, A.A. Hanna (eds) Proceedings of the 2nd International Conference on Africanized Honey Bees and Bee Mites. Root: Medina, OH.
Hyne, G.J., E. Guzman-Novoa, J.L. Rubio, D. Prieto-Merlos. 2003. Genotype by environment interactions in honey bee guarding behavior. Anim. Behav. 66: 459-67.
Hunter, L.A., J.A. Jackman, E.A. Sugden. 1993. Detection records of Africanized honey bees in Texas during 1990, 1991 and 1992. Southwest. Entomol. 18: 79-89.
Kerr, W.E. 1967. The history of the introduction of African bees to Brazil. S. Afr. Bee J. 39: 3-5
Kerr, W.E., S. de Leon del Rio, M.D. Barrionuevo. 1982. The Southern limits of the distribution of the Africanized honey bee in South America. Am. Bee. J. 122: 196-98.
Lobo, J. A. 1995. Morphometric, isozymic and mitochondrial variability of Africanized honeybees in Costa Rica. Heredity, 75(2): 133-141
Loper, G.M. 2002. Nesting sites, characterization and longevity of feral honey bee colonies in the Sonoran desert of Arizona: 1991-2000. In Erickson, E. R.E. Page, A.A. Hanna (eds) Proceedings of the 2nd International Conference on Africanized Honey Bees and Bee Mites. Root: Medina, OH. 86-96
Loper, G.M., J. Fewell, D.R. Smith, W.S. Sheppard, N. Schiff. 1999. Changes in the genetics of a population of feral honey bees (Apis mellifera L.) in S. Arizona after the impact of tracheal mites (Acarapis woodi), Varroa mites (Varroa jacobsoni) and Africanization. In Hoopingardner, R., and L. Conner (eds). Apiculture for the 21st Century. Wicwas: Cheshire CT. 47-51
McNally, L.C. and S.S. Schneider. 1992. Seasonal cycles of growth, development, and movement of the African honey bee, Apis mellifera scutellata, in Africa. Insectes Soc. 39: 167-79
McNally, L.C. and S.S. Schneider. 1996. Spatial distribution and nesting biology of colonies of the African honey bee Apis mellifera scutellata (Hymenoptera: Apidae) in Botswana, Africa. Environ. Entomol. 25: 643-52.
Moretto, G. 1997. La relación entre el comportamiento de limpiadura del nido de cría y el comportamiento de quitar los ácaros Varroa en las abejas melíferas africanizadas. Apiacta, 32(1): 17-20
Moretto, G., L.S. Gonçalves, D. De Jong, and M.Z. Bichuette. 1991. The effects of climate and bee race on Varroa jacobsoni Oud infestations in Brazil. Apidologie, 22(3): 197-203
Nogueira-Neto, P. 1964. The spread of a fierce African bee in Brazil. Bee World, 45(3): 119-121
Otis, G.W. 1991. Population biology of the Africanized honey bee. In Spivak, M. D.J.C. Flecher, M.D. Breed (eds). 1991. The “African” Honey Bee.Westview: Boulder CO. 213-34
Otis, G.W., O.R. Taylor, M.L. Winston. 2002. Colony Size affects reproductive attributes of African honey bees (Apis mellifera L.) In Erickson, E. R.E. Page, A.A. Hanna (eds) Proceedings of the 2nd International Conference on Africanized Honey Bees and Bee Mites. Root: Medina, OH.22-32
Prieto-Merlos, D. 2002. Confiabilidad de pruebas metodos para evaluar al comportamiento defensive y el tamano corporal en tres genotipos de abejas meliferas (Apis mellifera L.) MS Thesis. Natl. Univ. Mex., Mexico DF. 56 pp.
Quezada-Euán, J.J.G., C.M. Echazarreta, R.J. Patton. 1996. The distribution and range of expansion of Africanized honey bees (Apis mellifera) in the state of Yucatan, Mexico. J. Apic. Res. 35: 85-95
Quezada-Euán, J.J.G. and S.M. Hinsull 1995. Evidence of continued European morphometrics and mtDNA in feral colonies of honey bees (Apis mellifera) from the Yucatan Peninsula. J. Apic. Res. 34:161-66
Ratnieks, F. and P.K. Visscher. 1996. Agricultural impact of Africanized honey bees in Sinaloa, Mexico. Calif Agric, 50, 24-28.
Rinderer, T. E., J.A. Stelzer, B.P. Oldroyd, S.M. Buco, & W.L. Rubink. 1991. Hybridization between European and Africanized honey bees in the neotropical Yucatan peninsula. Science, 253(5017): 309-311
Rinderer T.E., B.P. Oldroyd, W.S. Sheppard. 1993. Africanized bees in the U.S. Sci. Am. 269:84-90
Rinderer, T.E., R.L. Hellmich, R.G. Danka, A.M. Collins. 1985. Male reproductive parasitism: a factor in the Africanization of European honey bee populations. Science 228:1119-21
Rinderer, T.E., A.M. Collins, R.L. Hellmich, R.G. Danka. 1987. Differential drone production by Africanized and European honey bee colonies. Apidologie 18:61-68
Ritter, W. and D. De Jong. 1984. Reproduction of Varroa jacobsoni O. in Europe, the middle East and tropical South America. Zeitschrift für Angewandte Entomologie, 98(1‐5): 55-57
Rubink, W.L., P. Luevano-Martinez, E.A. Sugden, W.T. Wilson, A.M. Collins.1996. Subtropical Apis mellifera (Hymenoptera: Apidae) swarming dynamics and Africanization rates in northeastern Mexico and southern Texas. Ann. Entomol. Soc. Am. 89: 243-51
Ruttner, F. 1988. Biogeography and Taxonomy of Honeybees. Springer: Berlin.
Schneider, S.S. 1995. Swarm movement patterns inferred from waggle dance activity of the Neotropical African honey bee in Costa Rica. Apidologie 26: 395-406
Schneider, S.S. and G. DeGrandi-Hoffman. 2002. The influence of worker behavior and paternity on the development and emergence of honey bee queens. Insectes Soc. 49:306-14
Schneider, S.S. and G. DeGrandi-Hoffman. 2003. The influence of paternity on virgin queen success in hybrid colonies of European and African honey bees. Anim. Behav. 65: 883-92.
Schneider, S.S., G. DeGrandi-Hoffman, and D. R. Smith. 2004. The African Honey Bee: Factors Contributing to a Successful Biological Invasion. Annual Reviews in Entomology 49: 351-376.
Schneider, S.S. and H.G. Hall. 1997. Diet selection and foraging distances of African and European-African hybrid honey bee colonies in Costa Rica. Insectes Soc. 44:171-87.
Schneider, S.S., L.C. McNally. 1992. Colony defense in the African honey-bee in Africa (Hymenoptera: Apidae) Environ. Entomol. 21:1362-70
Schneider, S.S., L.C. McNally. 1994. Waggle dance behavior associated with seasonal absconding in colonies of the African honey bee, Apis mellifera scutellata. Insectes Soc. 41: 115-27.
Schneider, S.S., L.C. McNally. 1993. Spatial foraging patterns and colony energy status in the African honey bee, Apis mellifera scutellata. J. Insect Behav. 6:195-210
Schneider, S.S., S. Painter-Kurt, G. DeGrandi-Hoffman. 2001. The role of the vibration signal during queen competition in colonies of the honey bee, Apis mellifera. Animal Behav. 61:317-22
Schneider, S.S., L.C. McNally. 1992. Factors influencing seasonal absconding in colonies of the African honey bee, Apis mellifera scutellata. Insectes Soc.39: 403-23
Schneider, S.S., L.J. Leamy, L.A. Lewis, G. Degrandi-Hoffman. 2003. The Influence of hybridization between African and European honey bees, Apis mellifera, on asymmetries in wing size and shape. Evolution. 57 (10), 2350-2364.
Schneider S.S., T. Deeby, D.C. Gilley and G. DeGrandi-Hoffman. 2004. Seasonal nest usurpation of European colonies by African swarms in Arizona, U.S.A. Insectes Sociaux 51: 359-364
Segura, J.A.L. 2000. Highly polymorphic DNA markers in an Africanized honey bee population in Costa Rica. Genet. Mol. Biol. 23:317-22
Sheppard, W.S., T.E. Rinderer, J.A. Maxxonli, J.A. Stelzer, H. Shimanuk. 1991. Gene flow between African- and European-derived honey bee populations in Argentina. Nature 349:782-84
Sousa, R.M., B.M. Fretas, Z.B. de Aruajo, A.E.E. Soares. 2002. Seasonal changes in Africanized honey bee (Apis mellifera L.) population of the Caatinga vegetation in N.E. Brazil. In Erickson, E. R.E. Page, A.A. Hanna (eds) Proceedings of the 2nd International Conference on Africanized Honey Bees and Bee Mites. Root: Medina, OH. 16-24
Spivak, M. and M. Gilliam. 1998. Hygienic behavior of honey bees and its application for control of brood diseases and varroa. Bee World, 79(4): 169-186
Spivak, M., D.J.C. Flecher, M.D. Breed (eds). 1991. The “African” Honey Bee.Westview: Boulder CO.
Stort, A.C., and L.S. Goncalves. 1991. Genetics of defensive behavior II. In Spivak, M. D.J.C. Flecher, M.D. Breed (eds). 1991. The “African” Honey Bee.Westview: Boulder CO.
Taylor, O.R. 1999. Displacement of European honey bee subspecies by an invading African subspecies in the Americas. In Hoopingardner, R., and L. Conner (eds). Apiculture for the 21st Century. Wicwas: Cheshire CT. 38-46
Taylor, O.R. 2003. Neotropical African (killer) bees. In Resh, V., R. Carde. (eds). Encyclopedia of Insects. Academic Press: New York. 776-78
Vandame, R., S. Morand, M.E. Colin, and L.P. Belzunces. 2002. Parasitism in the social bee Apis mellifera: quantifying costs and benefits of behavioral resistance to Varroa destructor mites. Apidologie, 33(5): 433-446
Vandame, R., M.E. Colin, S. Morand, and G. Otero-Colina. 2000. Levels of compatibility in a new host-parasite association: Apis mellifera/Varroa jacobsoni. Canadian journal of zoology, 78(11), 2037-2044.
Vergara, C., A. Dietz, A. Perez de Leon. 1993. Female parasitism of European honey bees by Africanized honey bee swarms in Mexico. J. Apic. Res. 32: 34-40
Villa, J.D., N. Koeniger, T.E. Rinderer. 1991. Overwintering of Africanized, European, and hybrid honey bees in the Andes of Venezuela. Environ. Entomol. 22:183-89
Villa, J.D., T.E. Rinderer, A.M. Collins. 1993. “Overwintering” of Africanized, European, and hybrid honey bees in the Andes of Venezuela. Environ. Entomol. 22: 183-89
Villa, J.D., T.E. Rinderer, J.A. Stelzer. 2002. Answers to the puzzling distribution of Africanized bees in the United States. Am. Bee J. 142:480-483
Visscher, P.K., R.S. Vetter, F.C. Baptista. 1997. Africanized bees, 1990-1995: Initial rapid expansion has slowed in the U.S. Calif. Agric. 51: 22-25
Winston, M.L. 1992. Killer Bees: The Africanized Honey Bee in the Americas. Harvard University Press: Cambridge MA
Zillikens, A., Z.L.P. Simoes, W. Engels. 1998. Higher fertility of queenless workers in the Africanized honey bee. Insects. Soc. 45:473-76
Thank you to Stan Schneider (Univ. of N. Carolina) for review of this article
