Authors: Philip A. Moore, Michael E. Wilson, John A. Skinner
Department of Entomology and Plant Pathology, the University of Tennessee, Knoxville TN
Originally Published: August 4, 2015
Introduction
The honey bee tracheal mite (Acarapis woodi) was first described in 1921 by Rennie, who believed the mite was the cause of the Isle of Wight Disease, after dissecting infected honey bees (Apis mellifera) from colonies on the island off the coast of England (Henderson and Morse 1990). Between 1905 and 1919, 90 percent of colonies on the island were killed in one of the most infamous honey bee epidemics in history (Adam 1968). Although the causative agent or complex of agents was never specifically identified, tracheal mites and Nosema are commonly cited (vanEnglesdorp and Meixner 2010). The disease spread to the mainland United Kingdom and continental Europe, which initiated a ban on imports of live bees into the United States in 1922 (Phillips 1923).
In 1980, tracheal mites were discovered in Mexico. By 1984, bees from a commercial beekeeping operation in Weslaco Texas tested positive for the parasite. In spite of efforts to restrict the distribution of the pest, including the destruction of 43,367 colonies, tracheal mites reached all major beekeeping states in less than five years (Mussen 2001). Exceedingly high colony losses reported by beekeepers in many parts of the US in 1986-1989 were attributed to tracheal mites (Furgala et al 1989, Bailey 1981). For example, when the pest first reached Pennsylvania, beekeepers with infested colonies lost 31 percent of their colonies overwinter, compared to their non-infested neighbors who lost just 11 percent (Frazier et al 1994). Tracheal mites have now been spread throughout Europe, Asia, parts of Africa and North and South America. It is not known whether Australia, New Zealand, or Scandinavian countries contain the pest (Denmark et al 2000; Hoy 2011).
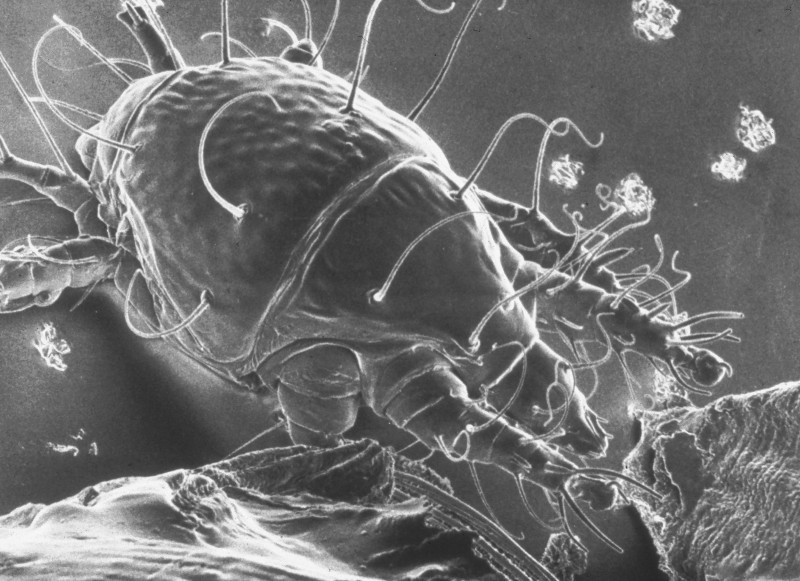
Figure 1: Tracheal mite as seem in a scanning electron micrograph (SEM). Credit: The Food and Environment Research Agency (Fera), Crown Copyright
Tracheal Mite Life History
Tracheal mites are oval shaped, between 125-174 micrometers long and 60-81 micrometers wide with a semi glossy white color, numerous setae (bristle like hairs), three sets of legs, and a long piercing mouthpart for feeding (Delfinado-Baker and Baker 1982). They are primarily found in the adult honey bee respiratory system that is composed of a network of tracheae tubes and have also been found in the air sacks of the abdomen and head (Giorani 1965) and externally at the base of the wing (Royce and Rossignol 1991). The tracheae initiate in large trunks at the spiracles (openings for air passage) and progressively branch out into smaller tracheoles that serve to carry air into and out of the honey bee, connecting to all tissues of the body. Female mites enter the first set of spiracles of adult bees and lay eggs in the large initial section of tracheae.
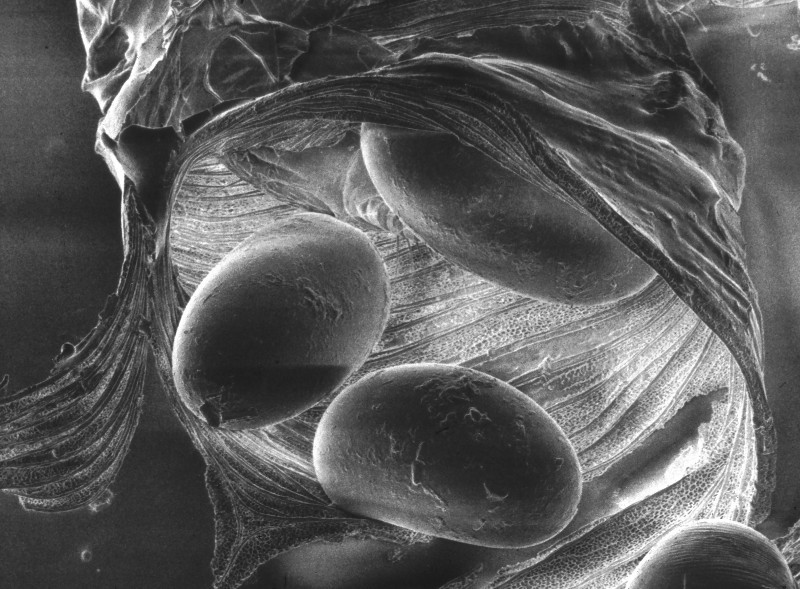
Figure 2: Tracheal mite eggs inside a trachea tube. Credit: The Food and Environment Research Agency (Fera), Crown Copyright
The female mite will lay 5-7 eggs over 3-4 days (Morgenthaler 1931). After 3-4 days the eggs hatch and progress from larva to nymph, and into the adult stage. Males develop to adulthood 11-12 days after hatching, while females develop in 14-15 days. Mating occurs in the same trachea in which it developed. All mite instars live within the tracheae, except for the brief period when a mated female disperses to find a new host. Female mites disperse when the bee host is at least 12 days old, typically between 15 to 25 days (Pettis and Wilson 1996), most often dispersing at night when the older foraging bees are in close contact with young bees (Pettis et al 1992). Mites exposed outside of the host will die within a few hours and therefore do not persist on comb or other colony and environmental components (Sammataro and Needham 1996; Sammataro et al. 2000).
The mated female leaves the tracheae by emerging from the spiracle and adhering to the tip of a hair on the bee’s thorax. Once another bee’s hair brushes against the original host, the mite attaches to the passing bee (Hirschfelder and Sachs 1952). Mites are attracted to the exhausted air of the spiracle and to specific hydrocarbons from the bee’s cuticle (Phelan et al 1991; McMullan et al 2010). Female mites are less attracted to older bees and rarely enter bees older than 4 days (Gary et al 1989). These older bees may not live long enough in the summer for the mite to complete its lifecycle.
Because of the time lag of invading a host, laying eggs, mite development, mating and the new reproductive females’ emergence, foraging workers bees (who contain the reproductive female mite) must contact young worker bees for successful transmission. Foraging worker bees rarely encounter young bees, due to division of labor and physical separation of tasks in the hive, except at night or periods when weather does not permit leaving the hive (Bailey and Perry 2001). When workers are unable to forage, such as during poor weather, mites are more able to find young bees and the rate of transmission increases (Bailey and Perry 2001).
Tracheal Mite Prevalence in the United States

Figure 3: USDA-ARS bee diagnostic lab percentage of tracheal mite infestation of all submitted samples for pest or disease analysis by year.
Figure 3 displays the prevalence of tracheal mites in diagnostic samples sent to the USDA-ARS bee lab in Beltsville Maryland. This shows a decline in tracheal mite prevalence since introduction into the US. This may indicate that current control and resistance techniques are effective in reducing the pest population or that the pest population is declining for some other reason. However, this data is not from a systematic sample and may contain sampling bias by location, reasons for sampling, or other unknown factors. It is therefore not completely representative of US honey bee colonies, but does indicate a reduction in prevalence of samples submitted for diagnosis to the USDA-ARS Beltsville bee lab, and reflects a decline in prevalence seen in other studies and observations. Current systematic honey bee pest surveys by USDA-APHIS do not measure tracheal mite prevalence.
Damage Caused by Tracheal Mites
Tracheal mites feed on bee hemolymph (fluid of the circulatory and lymphatic system similar to blood), which they access by piercing the wall of the trachea with their sharply pointed stylets (mouthpart). The mite then sucks the hemolymph through a short tube into the pharynx (Hirschfelder and Sachs, 1952). These mites, unlike Varroa mites, have not been shown to transmit other microorganisms through feeding. Individual bees are thought to die because mite populations build up in the tracheae, limiting or preventing air flow, or by damaging the tracheae during feeding (Shimanuki et al 1992).

Figure 4. Mortality spiral on a moderately infested colony during the winter/early spring period. Credit: McMullan and Brown 2009
Heavy mite population affects bee metabolism, and the ability of the winter cluster to regulate temperature (Skinner 2000). Tracheal mites can cause diminished brood area, smaller adult bee populations, loose winter clusters, increased honey consumption, which all combine to cause colony death (Figure 4; McMullan and Brown 2009; Komejli et al 1989). When over 30 percent of bees in a colony become infected, honey production is reduced and winter survival decreases (Furgala et al 1989). Bees confined over winter cause increased mite populations in the winter cluster. Colonies with greater than 40 infection frequently die over winter in the US and Canada (Furgala et al 1989; Otis and Scott-Dupree 1992). The colder and longer the winter, the more likely an infested colony will die.
Tracheal mites can infect all castes of adult honey bees. Drones have been found to harbor more mites than workers (Royce and Rossignol 1991; Darwicke et al. 1992). However since workers are available all year, they are the primary host. Queens infected with tracheal mites weigh less, although the effect on reproductive ability is not well understood (Camazine et al 1998). Commercially reared queens have commonly been found with tracheal mites, although the most recent survey of US queen producers found that only one of the twelve producers surveyed had some tracheal mite infected queens (Delaney et al 2010).
There are no reliable indicators or symptoms of mite infestation. Tracheal mites shorten the lifespan of adult bees and have been associated with disjointed “K” wings and bees crawling on the ground near a hive, unable to fly. Unfortunately, these symptoms are also associated with Nosema infection (Fries et al. 2013) and some viruses (de Miranda et al 2013). The only way to diagnose a tracheal mite outbreak is to examine honey bees for mites.
Detection of Tracheal Mites
Diagnosis of tracheal mite infestation is accomplished through microscope examination of the tracheae. The timing of sampling is important because the population of tracheal mites varies with the season. The greatest likelihood of detection is the late fall, winter, or early spring when bee populations are at the lowest and a high proportion of old bees are present, which have allowed for mites to reproduce. Infestation decreases in the summer when large bee populations dilute the mite population and bee turn over limits mite reproduction.
Bee age influences the detection of tracheal mites, therefore the location of sampling should be considered. Collect 50 bees from the frames of honey supers, the inner cover, or hive entrance, where older bees congregate. Drones tend to have higher mite abundance and should be collected as well, when available. Bees can be collected with a hand held modified insect vacuum or by scooping up bees directly into a wide mouth jar. Add the sample to a wide mouth jar and label the jar with the colony identity and date. Add 70% ethanol or freeze the jar to kill the bees (examination of tracheae is easier with no alcohol present). Bees should not be stored in alcohol for a long period because this will darken the tissue and obscure the tracheae.
If the bees are to be sent to the Beltsville Maryland USDA-ARS lab for diagnosis, the samples must be wet in alcohol. To ship through the postal service, samples are treated as if for Varroa. Collect 100 recently dead, dying, or older bees, and soak in 70% alcohol (ethyl, methyl, or isopropyl). Prior to shipping, pour off excess alcohol because postal services will not accept packages containing alcohol and pack into a leak proof container. For more information on submitting a sample for analysis see here. If a dissecting and compound microscope is available, dissection of the bees and examination of the tracheae is possible.
Dissection for Tracheal Mites
All 50 bees do not necessarily need to be sampled. A sequential sampling technique allows one to classify a low infestation (<10%) and high infestation (>10%), which will help determine the decision to treat with an acaricide (miticide). If the first 3 out of 7 bees sampled are infested, there is a high infestation and should be treated. If less than 3 bees are infested, continue sampling until 17 bees have been examined. After examining 17 bees, if only one is infested, the colony has a low infestation and does not need to be treated. If 5 of 17 bees are infested, then the colony is highly infested. If 2-4 bees are infested, continue sampling. In general, infestations lower than 20% do not require treatment, but that varies depending on the length and severity of winter (Frazier et al 2000).

Figure 4: Honey bee tracheae as seen after removing the head, first set of legs, and collar. Credit: Zach Huang
There are many techniques for examining bees for tracheal mites. The classical technique is to place the bee on its back and secure with a pin. Then, under a dissecting microscope, use a scalpel or razor blade to remove the bee’s head and first set of legs. The first ring of the thorax, called the collar, is then removed with forceps. This exposes the tracheal trunk. Using a fine pair of forceps and probe, remove the tracheae and carefully place it onto a microscope slide with a drop of glycerol or 85% lactic acid and cover slip.
Place the slide on a compound microscope. The tracheae of severely infected bees may have brown blotches or be black, obscured by numerous mites. Healthy tracheae will be cream or white. However, trachea may not always be discolored when mites are present, and discolored tracheae do not always contain mites. To view a video on this technique click here.

Figure 5: Healthy and infested tracheae tubes. Credit: The Food and Environment Research Agency (Fera), Crown Copyright
Control
Treatment for tracheal mites includes vaporizing menthol crystals and chemical miticides. Cultural control measures include resistant lines of bees, grease patties made from vegetable shortening and sugar, and proper apiary location. No biological controls currently exist. Be aware that some miticides marketed for Varroa control also control tracheal mites. However if treatments for Varroa are discontinuing or switched to a different chemical product, the beekeeper may no longer be treating for tracheal mites as well. Since miticides are often only tested for efficacy against Varroa, the effectiveness of a given chemical control against tracheal mites may be unclear and will not be present on the product label.
Cultural Control
Honey bees show considerable variation in phenotypic susceptibility to tracheal mites (Gary and Page 1987, Gary et al. 1990, Page and Gary 1990, Milne et al. 1991, Szabo et al. 1991, Otis and Scott-Dupree 1992, Danka et al. 1995, Lin et al. 1996, Guzman et al. 1998, Nasr et al 2001). Among commercially available bee strains, Buckfast bees have shown resistance to tracheal mites (Milne et al. 1991, Danka et al. 1995, Lin et al. 1996) as have Russian bees (de Guzman et al 2002; de Guzman et al 2005).
Resistance to tracheal mites is heritable and can be passed from one generation to the next (Nasr et al 2001) and is likely brought about through grooming behaviors of bees. Both grooming between bees and self grooming by dancing in particular are associated with mite resistance (Pettis and Pankiw 1998; Danka and Villa 1998). Another possibility is that resistance is achieved by some bees being less attractive to mites due to their odor (Phelan et al. 1991; van Engelsdorp 1995). It is likely that a combination of host recognition by mites and removal of mites through grooming both translate into fewer mites entering the tracheae of resistant bees compared to susceptible bees.
Grease patties are made from mixing two parts white granulated sugar to one part hydrogenated vegetable shortening by volume. A four ounce (113 g) patty is placed on the top bars at the center of the broodnest where it is most likely to contact the most bees. The shortening appears to disrupt the questing female mites as they search for a new host (young bees) (Sammataro and Needham 1996; Sammataro et al. 1994). Because young bees are emerging continually, the patty must be present for an extended period. Application should be made in fall and early spring when mite levels are increasing.
Because heat is associated with mite mortality (Harbo 1993), placing hives in direct sunlight may limit mite population growth and shading tends to accelerate it (de Guzman unpublished data; vanEngelsdorp and Otis 2001).
Chemical Control
As of this writing, the organic chemical formic acid (Mite Away II™) is registered for tracheal mite control. Other widely used treatments against Varroa mites may also be affective against tracheal mites, but are not labeled for tracheal mite control since their primary target for study was Varroa mites.
Based on research by Giordani (1977), menthol was tested and registered for use in the US and Canada. Menthol is extracted from the mint plant Mentha arvensis and is sold as crystals inside of a nylon mesh sac, which is placed on the top bars of frames, as the labeled product, Mite-A-Thol™. This application is temperature dependent with too cool temperatures resulting in reduced volatilization and a too high temperatures forcing bees out of the colony. If bees are hanging outside of the colony entrance in treated colonies, the crystals should be removed until temperatures drop.
Summary
Tracheal mites are a wide spread and serious pest of western honey bees. Current genetic resistance and control of the pest appears to be effective in the US. However high winter loss rates in the last decade and limited infection data leaves conclusions of the current damage caused by tracheal mites to be unknown. Unfortunately, laboratory diagnosis is the only way to determine if a colony is infested. A vigilant beekeeper should not become complacent; do not assume tracheal mites are under control or do not exist in apiaries if an effective monitoring protocol is not in place.
References:
Bailey, L., B. V. Ball. 1991. Honey bee pathology. Academic Press; London UK
Camazine, S., I. Cakmak, K. Cramp, J. Finely, J. Fisher, M. Frazier. 1998. How healthy are commercially-produced US honey bee queens? American Bee Journal 138: 677-680
Danka, R. G. and J. D. Villa. 1998. Evidence of autogrooming as a mechanism of honey bee resistance to tracheal mite infestation. J. Apic. Res 39–46. 37.
Danka, R. G., J. D. Villa, T. E. Rinderer, and G. T. Delatte. 1995. Field test of resistance to Acarapis woodi (Acari: Tarsonemidae) and of colony production by four stocks of honey bees (Hymenoptera: Apidae). J. Econ. Entomol 584–591. 88.
Dawicke, B.L., G. W. Otis, C. Scott-Dupree, M. E. Nasr. 1992. Host preference of the honey-bee tracheal mite (Acarapis woodi (Rennie)). Experimental and Applied Acarology 15: 83-98
de Guzman, L. I., T. E. Rinderer, G. T. Delatte, J. A. Stelzer, G. Beaman, V. Kuznetsov. 2002. Resistance to Acarapis woodi by honey bees from Far-eastern Russia. Apidologie 33(4): 411-415
de Guzman, L. I., T. E. Rinderer, M. Bigalk, H. Tubbs, S. J. Bernard. 2005. Russian honey bee (Hymenoptera: Apidae) colonies: Acarapsis woodi (Acari: Tarsonemidae) infestations and overwintering survival. Journal of Economic Entomology 98 (6): 1796-1801
de Miranda, J. R., L. Bailey, B. V. Ball, P. Blanchard, G. Budge, N. Chejanovsky, Y. P. Chen, L. Gauthier, E. Genersch, D. de Graff, M. Ribiere, E. Ryabov, L. de Smet. J.J.M. van der Steen. 2013. Standard methods for virus research in Apis mellifera. In V. Dietman, J. D. Ellis, P. Neuman (Eds.) The Coloss Beebook, volume II: standard methods for Apis mellifera pest and pathogen research. Journal of Apicultural Research 52(4)
Delaney, D. A., Keller, J. J., Caren, J. R., & Tarpy, D. R. (2010). The physical, insemination, and reproductive quality of honey bee queens (Apis mellifera L.).Apidologie.
Delfinado-Baker, M. 1984. Acarapis woodi in the United States. American Bee Journal. 124:805-806.
Delfinado-Baker, M. E. W. Baker. 1982. Notes on honey bee mites of the genus Acarpis Hirst (Acari: Tarsonemidae). International Journal of Acarology 8: 211-226.
Denmark, H.A., H.L. Cromroy, M.T. Sanford. 2000. Honey bee tracheal mite, Acarapis woodi (Rennie) (Arachnida: Acari: Tarsonemidae). SPI Entomology Circular, 267. University of Florida, IFAS Extension.
Eckert, J.E. 1961. Acarapis mites of the honey bee, Apis mellifera Linnaeus. Journal of Insect Pathology 3: 409-425
Fries, I., M. P. Chauzat., Y. P. Chen, V. Doublet, E. Genersch, S. Gisder, M. Higes, D. P. McMahon, R. Martin-Hernandez, M. Natsopoulou, R. J. Paxton, G. Tanner, T. C. Webster, G. R. Williams. 2013. Standard methods for nosema research. In V. Dietman, J. D. Ellis, P. Neuman (Eds.) The Coloss Beebook, volume II: standard methods for Apis mellifera pest and pathogen research. Journal of Apicultural Research 52(1)
Furgala, B., Duff, S., Aboulfaraj, S., Ragsdale, D., & Hyser, R. (1989). Some effects of the honey bee tracheal mite (Acarapis woodi Rennie) on non-migratory, wintering honey bee (Apis mellifera L.) colonies in east central Minnesota. American bee journal (USA).
Furgala, B., S. Duff, S. Aboulfara, D. Ragsdale, R. Hyser. 1989. Some effects of the honey bee tracheal mite (Acarapis woodi Rennie) on non-migratory, wintering honey bee (Apis mellifera L.) colonies in east central Minnesota. American Bee Journal 129: 195-197.
Giordani, G. 1961. Recherches au laboratoire suyr Acarapis woodi Rennie, agent de l’acariose des abeilles (Apis mellifera L) Note 1. Bulletin Apicole, V: 33-48.
Giordani, G. 1961. Recherches au laboratoire suyr Acarapis woodi Rennie, agent de l’acariose des abeilles (Apis mellifera L) Note 4. Bulletin Apicole, VII: 159-175.
Gary, N. E. and R. E. Page. 1987. Phenotypic variation in susceptibility of honey bees, Apis mellifera, to infestation by tracheal mites, Acarapis woodi. Exp. Appl. Acarol 291–305. 3.
Gary, N. E., R. E. Page, R. A. Morse, C. E. Henderson, M. E. Nasr, and K. Lorenzen. 1990. Comparative resistance of honey bees (Apis mellifera L.) from Great Britain and United States to infestation by tracheal mites (Acarapis woodi). Am. Bee J 667–669. 130.
Guzman, L. I de, T. E. Rinderer, and G. T. Delatte. 1998. Comparative resistance of four honey bee (Hymenoptera: Apidae) stocks to infestation by Acarapis woodi (Acari: Tarsonemidae). J. Econ. Entomol 1078–1083. 91.
Harbo, J. 1993. Field and laboratory tests that associate heat with mortality of tracheal mites. Journal of Apicultural Research 32 (3-4): 159-165
Hoy, M. A. 2011. Agricultural acarology: introduction to integrated mite management. CRC Press; Florida, USA. 303-308
Lin, H., G. W. Otis, and C. Scott-Dupree. 1996. Comparative resistance in Buckfast and Canadian stocks of honey bees (Apis mellifera L.) to infestation by honey bee tracheal mites (Acarapis woodi (Rennie)). Exp. Appl. Acarol 87–101. 20.
McMullan, J. B., & Brown, M. J. (2009). A qualitative model of mortality in honey bee (Apis mellifera) colonies infested with tracheal mites (Acarapis woodi). Experimental and Applied Acarology, 47(3), 225-234.
McMullan, J.B., R. D’Ettorre, M. J. F. Brown. 2010. Chemical cues in the host-seeking behavior of tracheal mites (Acarapis woodi) in honey bees (Apis mellifera mellifera). Apidology 41: 568-578
Milne, C. P., G. W. Otis, F. A. Eischen, and J. M. Domaier. 1991. A comparison of tracheal mite resistance in two commercially available stocks of honey bees. Am. Bee J 713–718. 131.
Nasr, M. E., Otis, G. W., & Scott-Dupree, C. D. (2001). Resistance to Acarapis woodi by honey bees (Hymenoptera: Apidae): divergent selection and evaluation of selection progress. Journal of economic entomology, 94(2), 332-338.
Otis, G. W. and C. D. Scott-Dupree. 1992. Effects of Acarapis woodi on overwintering colonies of honey bees (Hymenoptera: Apidae) in New York. J. Econ. Entomol 40–46. 85.
Otis, S. W., C. D. Scott-Dupree. 1992. Effects of Acarapis woodi on overwintering colonies of honey bees (Hymenoptera: Apidae) in New York. Journal of Economic Entomology 85: 40-48
Page, R. E. and N. E. Gary. 1990. Genotypic variation in susceptibility of honey bees (Apis mellifera) to infestation by tracheal mites (Acarapis woodi). Exp. Appl. Acarol 275–283. 8..
Pettis, J. S. and T. Pankiw. 1998. Grooming behaviour by Apis mellifera L. in the presence of Acarapis woodi (Rennie) (Acari: Tarsonemidae). Apidologie 241–253. 29.
Pettis, J. S. W.T. Wilson, F. A. Elschen. 1992. Nocturnal dispersal by female Acarapis woodi in honey bee (Apis mellifera) colonies. Experimental and Applies Acarology 15:99-108
Pettis, J. S., W. T. Wilson. 1996. Life history of the honey bee tracheal mite (Acari:Tarsonemidae). Arthropod Biology 89 (3): 368-374
Phelan, P. L. Smith, A. W., G. R., Needham. 1991. Mediation of host selection by cuticular hydrocarbons in the honey bee tracheal mite Acarapsis woodi (Rennie). Journal of Chemical Ecology 17:463-473.
Phelan, P. L., A. L. Smith, and G. R. Needham. Mediation of host selection by hydrocarbons in the honey bee tracheal mite Acarapis woodi (Rennie). J. Chem. Ecol 463–473. 17.1991.
Phillip, E. F. 1923. The occurance of diseases of adult honey bees. II. US Department of Agriculture #287
Royce, L. A., Rossignol, P.A. 1991. Sex bias in tracheal mite [Acarapis woodi (Rennie)] infestation of honey bees (Apis mellifera L.) Bee Science 1: 159-161
Sammataro, D. Needham, G.R. 1996. Host-seeking behavior of tracheal mites (Acari:Tarsonemidae) on honey bees (Hymenoptera: Apidae). Experimental and Applied Acarology 20: 212-136
Sammataro, D. U. Gerson, G. R. Needham. 2000. Parasitic mite of honey bees: life history, implications and impact. Annual Review of Entomology 45:519-548
Skinner, A. J. 2000. Impacts of tracheal mites (Acarapis woodi (Rennie)) on the respiration and thermoregulation of overwintering honey bees in a temperate climate. Masters Thesis. University of Guelph; Guelph, Ontario, Canada. 186p
Szabo, T. L., L. P. Lefkovitch, and K. J. Clark. 1991. Comparative resistance of honey bees from a closed population to infestation by tracheal mites. Am. Bee J 643–645. 131.
van Engelsdorp, D. 1995. A field evaluation of nine genetic lines of honey bees for tracheal mite resistance and other economically important traits. M.S. Thesis, University of Guelph, Guelph, Ontario, Canada.
vanEngelsdorp, D. Otis, G.W. 2001. Field evaluation of nine families of honey bees for resistance to tracheal mites. Canadian Entomology 133:793-803.
