
A National Research and Extension Initiative to Reverse Pollinator Decline
This is part of an ongoing series of updates from the Managed Pollinator CAP. Additional installments can be found at the:
More information about the CAP can be found at:
CAP Updates: 18
by JIM FRAZIER, CHRIS MULLIN, MARYANN FRAZIER and SARA ASHCRAFT Department of Entomology; Penn State University, 501 ASI Building, University Park, PA
Jointly published in the American Bee Journal and in Bee Culture, August 2011.
Introduction
Do pesticides cause CCD? This is a question that beekeepers have been asking ever since CCD hit the national scene in 2007. In a series of articles in the American Bee Journal, Randy Oliver has done an exceptional job of distilling much of the recent research results into a meaningful update for beekeepers relative to this question (Oliver, R. 2010; See also Scientific Beekeeping.com). Researchers too have been asking this question and the CAPS project has specific objectives to investigate the factors responsible for CCD. In a 2008 American Bee Journal article we gave our initial results of the first 108 samples analyzed for pesticide levels in pollen, beebread, and wax and indicated that the levels found were reason for concern about pesticide interactions, sub-lethal impacts, and interactions with other stressors (Frazier et al, 2008). Here we report some of our more recent progress on these topics and include results from not only the CAPS project efforts, but also from other researchers in addressing these questions.
What can we say about honey bee exposure to pesticides?
One of the first responses to the CCD eruption in 2007, was the immediate sampling of collapsing colonies across the US by the first cooperating group of researchers from university, state departments of ag and the USDA. Soon afterwards a migratory beekeeper study was initiated to follow selected migratory beekeepers from Florida through Maine and to sample their colonies after each stop along the way. It was from these studies that over 800 samples of bees, pollen, and wax have been analyzed for the presence of 171 different pesticides. We found that the 350 pollen samples contained at least one systemic insecticide 60% of the time and nearly half had the miticides fluvalinate and coumaphos, as well as the fungicide chlorothalonil. In bee-collected pollen we found chlorothalonil at levels up to 99 ppm and the insecticides aldicarb, carbaryl, chlorpyrifos and imidacloprid, fungicides boscalid, captan and myclobutanil, and the herbicide pendimethalin at 1 ppm levels along with chlorothalonil. The pollen samples contained an average of 6 different pesticides each with one sample containing 39 different pesticides. Almost all comb and foundation wax samples (98%) were contaminated with up to 204 and 94 ppm, respectively, of fluvalinate and coumaphos, and lower amounts of amitraz degradates. We concluded that the 98 pesticides and metabolites detected in mixtures up to 214 ppm in bee pollen alone represented a remarkably high level for toxicants in the food of brood and adults. While exposure to many of these neurotoxicants elicits acute and sublethal reductions in honey bee fitness, the effects of these materials in combinations and their direct involvement in CCD remain to be determined.
Two other studies have measured multiple factors associated with CCD and non-CCD colonies across the US to see what risk factors were predictive of CCD (vanEnglesdorp, et al, 2009, 2010). The first study looked at one factor at a time among 61 variables as potential causes of CCD and found that no one factor could account for CCD. The second study borrowed from a proven approach used in epidemiological studies for unknown diseases, incorporating all types of factors that might be associated with the phenomenon and then subjecting them to a statistical approach of classification and regression tree analysis known as CART (Saegerman, et al., 2004). Using 55 different variables and determiing their relationships and interactions to CCD indicated that factors measuring colony stress (e.g., adult bee physiological measures, such as fluctuating asymmetry or mass of head) were important discriminating values, while six of the 19 variables having the greatest discriminatory value were pesticide levels in different hive matrices. These pesticide levels included coumpahos in brood, esfenvalerate in wax, coumaphos in wax, iprodione in wax, docofol in beebread, and chlorothalonil in wax. Coumaphos levels in brood had the highest discriminatory value of 100% and was highest in control (healthy) colonies. This may seem surprising, yet we do not know the timeliness of treatments for Varroa in these colonies, or if the bees have been selected for increased pesticide tolerance, either one of which could account for this outcome. While this study used an unbiased analysis of multiple factors that might be associated with CCD, the results certainly indicate that pesticides are very likely involved and that interactions with other stressors are very likely factors contributing to CCD and the decline of honeybee health.
Although our work represents the largest data set of pesticides in honey bee colonies to date, and was drawn from samples collected across 23 states and a Canadian province, it was not the product of a well designed systematic survey of honey bee colonies in the US. It thus does not give us a clear picture of the current state of pesticide residues in honey bee colonies. Such a study is critically needed yet we know of no current plans to accomplish this expensive task. In addition, the number of pesticides registered for use in the US is over 1200 active ingredients distributed among some 18,000 products, which makes the chemical use landscape for US beekeepers very different from those in other countries such as France, where some 500 chemicals are registered or in England where fewer than 300 are registered (Chauzat et al. 2010: Thompson, personal communication). Studies of pesticide contamination in bee colonies in other countries such as France, Germany, the Netherlands, or Belgium, thus may not tell us much about the likelihood of contamination of bee colonies in the US (Chauzat et al, 2010; Genersch et al. 2010; Tennekes, 2010; Nguyen, et al. 2010). Pesticide exposure for migratory colonies is likely very different from that of stationary colonies, and perhaps also very different from that of colonies kept by organic beekeepers, yet this also is not well documented. Pesticide use records are complete only for the state of California, in other states data are currently unavailable, or vary limited in scope (Grube et al, 2011). There are, thus many unanswered questions regarding pollinator exposure to pesticides. We do not currently have an accurate picture of what pesticides are used, where and in what amounts, nor do we have accurate measures of just what the maximum exposure is in agricultural or urban settings on blooming plants. Once contaminated pollen is collected, the potential transformations of pesticides in bee bread and royal jelly are also currently unknown. Clearly the potential for pesticide involvement in declining honey bee health is far from being understood, and it is clearly too early to discount them as key factors associated with CCD.
What can we say about the risk assessment of pesticides on bee health?
There is much truth in the adage ‘you are what you eat.’A parallel in pesticide analysis is ‘you only find what you look for.’ Our approach in documenting pesticides in apiary samples (Mullin et al., 2010) has been to search for a wide sweep of pesticides (> 200) that are used frequently in hives and around bees where they forage. For many published studies that document pesticide residues, this has not been the case, and more emphasis has been placed on the neonicotinoid imidacloprid and other systemic insecticides with high bee toxicity. A focused study on one pesticide or a single class of chemicals allows for use of a more sensitive method of analysis, while an affordable method that detects many pesticides from widely different chemical classes is compromised by not attaining the lowest limit of detection (LOD) for every pesticide analyzed. We desired a more complete assessment of the toxic pesticide burden that bees encounter instead of a biased approach to search for only chemicals renowned for their bee toxicity. A caveat of this approach is that the attainable LOD for a focused method will generally be lower; the more chemically variable and greater number of pesticides in the screen increases costs of analysis while reducing, at least for some pesticides, the sensitivity of their detection (increasing LOD). Nevertheless, we chose an analysis that incorporates hive miticides and their metabolites in addition to a large number of potential pesticides from their foraging arena as a better way to measure potential sources of risk for honey bees.
Assessing the risk of pesticides and their metabolites requires a sensitive method for their analysis. However, is the most sensitive LOD the primary criteria for choosing a method of analysis? It may be if your major goal is to find a particular chemical. The lower the LOD, the more frequently it is detected in the samples analyzed. Thus, the percentage of samples with detections for a given pesticide increases with a LOD at parts per trillion (ppt) > parts per billion (ppb) > parts per million (ppm). However, risk assessors are more concerned about choosing methods that allow you to predict hazards and risks of exposure at levels above the no observable effect level (NOEL) or lowest observed effect level (LOEL). For bee foods and known acute toxicity or behavioral effects, and chronic sublethal effects on longevity and reproduction, generally a LOD greater than 1ppb is used which is sufficient even for the most toxic pesticides such as imidacloprid.
Assessing the risk of a pesticide to bees uses the effects after exposure such as the acute LD50 (lethal dose for 50% of treated bees) and long-term chronic or sub-lethal EC50 (effective concentration that reduces by 50% the growth, learning, longevity etc. of treated bees). The risk of exposure is predicted by both frequency and mean residue amounts in pollen, nectar, water and wax, and the persistence (time to remove 50% = half-life) and fate (degradation and metabolism rates) of the pesticide in the hive or exposed bee. Knowing the physicochemical properties of a pesticide active ingredient (octanol (oil)/water partition coefficient, water solubility, vapor pressure) will aid in predicting routes of exposure and the potential for bioconcentration. For example, the systemic imidacloprid in comparison to the miticide fluvalinate is about 10,000,000 times less soluble in oil than in water and greater than 700,000 times more water soluble. Thus, fluvalinate would be predicted to persist in the beeswax and fat tissues of bees, while imidacloprid would be ‘washed’ more readily out of the hive or be excreted by the bees.
Are neonicotinods the major pesticide risk for bees?
Systemic neonicotinoid use has greatly increased recently through transgenic seed treatments and use on many other major crops, ornamentals, turf and in structural pest control. Bee kills in France, Germany and the US have been associated with imidacloprid- and clothianidin-treated seeds (Minister of Ag, 2008). Acute LD50s average 28 and 24 ng/bee respectively, for imidacloprid and clothianidin, although sublethal effects have been reported at much lower levels (Decoutrye et al. 2004). Generally, the lowest observed sublethal effects for imidacloprid in the lab are at 1 ng/bee which is equivalent to 10 ppb for an average 100 mg bee. Achieving a 10 ppb dose would require consuming pollen with residues of 250 ppb imidacloprid at a consumption rate of 4 mg pollen/day (4% of bee’s body weight). This high residue level is never found when label-rates of Gaucho are used as a seed treatment (generally 1-5 ppb in pollen). Nectar residues of imidacloprid are usually less than in pollen, although more is consumed over the bee’s life. However, even if a forager ingests 10% of their body weight in nectar per day, it would require 100 ppb of imidacloprid in the nectar to achieve a 10 ppb dose per day, regardless of the high turnover rate of this water-soluble insecticide in the bee. Imidacloprid is known to be rapidly metabolized and is excreted by adult bees with a half-life of about 5 hours (Suchail et al., 2004). This means that more than double the above doses of imidacloprid in the food is required to maintain a body level that keeps up with its rapid clearance from the bee. It is unlikely that doses of neonicotinoids from routine systemic seed treatments will attain the necessary > 100 ppb levels in pollen or nectar to acutely impair honey bees. Dusts from im-properly formulated or applied seed treatments, however, (Minister of Ag, 2008) or guttation water from glandular exudations on treated plants (Girolami et al, 2009) do have the necessary high residues levels to directly kill bees (Wallner, 2009).
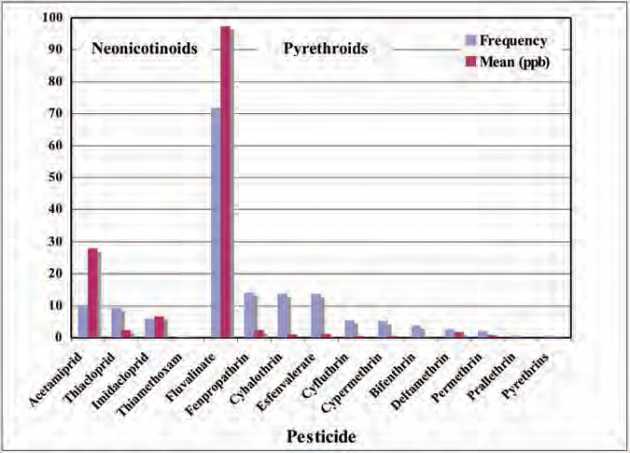
Figure 1. Frequency and mean residue amounts (non-detects = 0 ppb) of neonicotinoids and pyrethroids in 503 pollen samples.
Our residue results based on 1120 samples which include Mullin et al. (2010) and subsequently more than 230 additional samples do not support sufficient amounts and frequency of imidacloprid in pollen to broadly impact bees. For all samples, only 41 (3.7%) contained imidacloprid above the 2 ppb LOD with a mean residue of 12.3 ppb (scoring non-detects at 0 ppb). Among the other neonicotinoids, 66 detections (5.9%) were found for acetamiprid, 59 (5.3%) for thiacloprid, 3 (0.3%) for thiamethoxam, 2 (0.2%) for clothianidin and 0 for dinotefuran, and 9.9% of samples contained at least one neonicotinoid, mostly the less toxic acetamiprid and thiacloprid. This is in contrast to pyrethroids which were found in 79.4% of samples at 36-times higher amounts than the neonicotinoids, on average. For the 503 pollen samples that included some mixtures such as pollen with some nectar, wax etc, or whole anthers, which would maximize neonicotinoid levels, only 15.3% contained any neonicotinoid (Figure 1). The mean neonicotinoid residue was 37 ppb (scoring non-detects as 0 ppb), of which only 6.7 ppb was imidacloprid. Pyrethroids, by comparison, were present at a mean residue of 106 ppb and a frequency of 80.3% in pollen samples (Figure 1). These included fenpropathrin (LD50 = 50 ng/bee), cyhalothrin (79 ng/bee), cyfluthrin (22 ng/bee), bifenthrin (15 ng/bee), deltamethrin (50 ng/bee) and prallethrin (28 ng/bee); all of which are similar in acute bee toxicity to imidacloprid (LD50 = 28 ng/bee) and clothianidin (24 ng/bee). Indeed, if a relative hazard to honey bees is calculated as the product of mean residue times frequency detected divided by the LD50, the hazard due to pyrethroid residues is three-times greater than that of neonicotinoids detected in pollen samples (Figure 2).
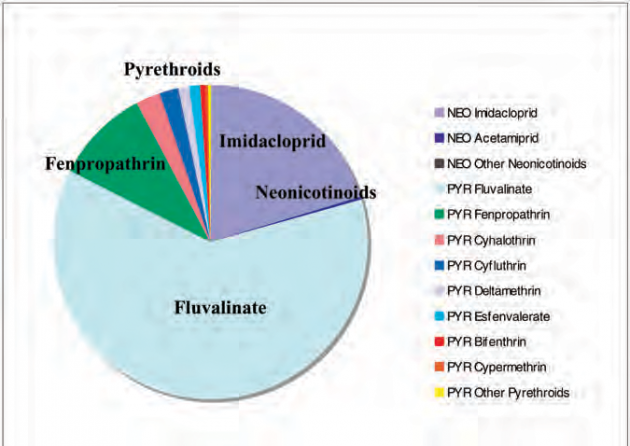
Figure 2. Relative hazard to honey bees of pyrethroids and neonicotinoids detected in 503 pollen samples estimated by (mean detection X frequency)/LD50.
The LOD has great bearing on the frequency of detections for a particular pesticide, with frequency increasing with decreasing LOD. If the most important factor for risk assessment is the mean residue level, this only slightly increases for an increase in LOD. For example, our 503 pollen samples had only 30 imidacloprid detections (mean of 6.7 ppb overall with non-detects scored 0 ppb). If our non-detects are scored as 0.1 ppb anticipated to be detected with a highly sensitive analysis, the mean ppb for the 503 samples would be < 6.8 ppb. Scoring non-detects at our LOD of 2 ppb would only increase the mean detection to < 8.6 ppb imidacloprid overall. These modest residue increases would not be significant for a consideration of the exposure of bees to imidacloprid.
What about pyrethroids as major bee toxicants?
Pyrethroids bioaccumulate in wax and bees due to their high fat solubility in contrast to neonicotinoids. In wax, 312 of 340 samples contained pyrethroids versus 2 with imidacloprid and 4 with thiacloprid, with the average pyrethroid residue content > 64,000 times higher than the total neonicotinoid. While fluvalinate prevailed (307 detections), many other detections of esfenvalerate (50), fenpropathrin (43), bifenthrin (37), cypermethrin (28), cyfluthrin (26), pyrethrins (16), cyhalothrin (13), deltamethrin (8) and permethrin (8) were found. A similar analysis for residues in 241 bee, brood and queen samples showed only 4 samples with neonicotinoids, two from bee kill incidences correlated with imidacloprid and thiamethoxam/clothianidin, respectively. The two other samples contained low amounts acetamiprid and thiamethoxam. Even with the higher neonicotinoid residues due to bee kills, a dozen pyrethroids distributed within 70% of our bee samples had a mean residue (non-detects = 0 ppb) of 357 ppb, 178 times greater than the 2 ppb for the neonicotinoids. Pyrethroid prevalence and persistence in the hive thus likely has more consequences for colony survival than the water-soluble neonicotinoids. The only other major insecticide detected in our hive samples with high toxicity was the organophosphate chlorpyrifos (LD50 = 122 ng/bee) in 42.6% of samples with an average detection of 36.3 ppb. This OP degrades more rapidly and is less persistent than pyrethroids. However, higher residues of the less toxic neonicotinoids acetamiprid and thiacloprid (Iwasa et al., 2004) or of pyrethroids (Pilling and Jepson, 1993; Johnson et al., 2011) in pollens with even higher amounts of fungicides may have considerable impact on bee health via their synergistic combinations. Pyrethroids disable foraging of bees at levels of 9 ng permethrin per bee (90 ppb) Cox et al. 1984) and 2.5 ng deltamethrin per bee (vanDame et al. 1995), which is of a potency similar to that of imidacloprid.
The in-hive miticide fluvalinate is one of the most fat-soluble or lipophilic of pyrethroids, with a water solubility less than 12 ppb or 12 µg/liter (EPA-OPP 2005), and is functionally insoluble in water or sucrose solutions without added solvents, surfactants or other formulation aids. Many acute toxicity bioassay results have been reported for this pesticide, with LD50s ranging from 65.85 down to 0.2 µg/bee for honey bees (Atkins et al. 1981, EPA-OPP 2005). To our knowledge, this is the most variable LD50 result noted among pesticide bioassays on bees, and most likely indicates that some of these bioassays were conducted, particular those for oral toxicity, without this pyrethroid being truly in solution. This highly non-polar chemical can adsorb to plastic or even glass walls of solution containers or application vessels in lieu of sufficient solubilizer additions, leading to extraneous results.
Sub-Lethal impacts of pesticides; a new arena of research
If we acknowledge that multiple pesticide residues in bee collected pollen are “typical”, and consider the number of possible impacts of ingesting this pollen, first by nurse bees, then by brood and finally by the queen, it is not surprising that we have not yet determined all of the possible outcomes. Almost all studies to date have focused on the action of a single pesticide so that very few combinations have been studied. We feel that this is a major limitation to our current level of understanding of pesticide impacts on bees.
When bees are exposed to a toxic dose of pesticides, dead bees surrounding the hive entrance are an obvious result. What is not so obvious, are the consequences of lower doses of one or more pesticides that may be encountered while foraging, or from collected pollen and nectar brought back to the hive. It is these sub-lethal impacts that have become the focus of much of the current research on pesticides. Many studies have documented impacts of low levels of pesticide exposure that when ingested for longer periods of time result in more chronic impacts. Such actions have been reviewed for many beneficial insects as well as for pollinators (Desneux, et al, 2007). The impacts of such consequences have been many and varied and have led to the loss of many kinds of beneficial insects not just pollinators. One such example is the loss of important insect biocontrol agents in apple orchards, which has allowed the emergence of new pests in the absence of their natural enemies; all unintended outcomes of sublethal effects on different insects with different sensitivities (Agnelo et al, 2009). What are the parallel kinds of impacts on honey bees and other native pollinators? The answers are only beginning to emerge, but current research is finding some surprising results. For honey bees low levels of pesticides have been shown to reduce associative learning of individual bees in laboratory studies using the proboscis extension response (Decourtye et al, 2004), altering maze learning performance in free-flying bees (Decourtye, et al. 2010) and the loss of foraging efficiency in radio tagged bees, (Decourtye, et al. 2011). The precocious foraging of nurse bees from IGR insecticides is also documented (Thompson et al. 2007). These changes in learning and behavior can potentially alter normal colony level functions, yet colony-level impacts remain to be verified.

Figure 3. A honey bee gathering corn pollen, a common source of neonicotinoid residues in teh Midweestern Us.
Honey bee larvae reared in cells contaminated with the miticides fluvalenate or coumaphos show a reduced developmental rate and delayed adult emergence along with reduced adult longevity (Wu et al, 2011). These effects can have multiple consequences for the colony including increased developmental time for Varroa mites, reduced colony population dynamics and build up, as well as potential shifts in worker division of labor. Whether or not the pesticides associated with wax in the CART study (aboce) have similar impacts on larvae remains to be determined. Fungicides have long been known to synergize with some pesticides in laboratory toxicity bioassays (Iwasi et al, 2004). More recently, we have determined that combinations of formulated pesticides and fungicides fed to either adult worker bees or to larvae can have synergistic effects on mortality. What happens when 3 or 4 or 5 different pesticide mixtures are ingested by honey bee larvae or adults for substantial periods of time? Studies to determine some of these impacts have been completed and will be published later this year.
What can beekeepers do to decrease the potential pesticide exposure and/or respond to a pesticide incident?
Honey bees are supremely good at finding pollen and nectar sources in their environments. The average foraging range of a single colony is thought to be a 3.75 mile radius most of the time with trips up to 6.75 miles in times of great need (Figure 4). In a typical US setting this range includes 28,000+ acres with a lot of different plants, some of which are in bloom at any given time, and some of which may have been treated with pesticides. If the average colony can find the most nutritious nectar source within a two hour window, then the incoming flow of nectar and pollen is very dynamic indeed (Seeley, 1995). How much of this foraging range do you normally consider as the pollen and nectar sources for your colony? If this includes areas beyond your direct control, then this is an important dynamic for you to consider in your colony’s potential health. Pesticide applications are made by many people for lots of reasons, but with these beyond your control, the typical colony is at the mercy of these events in their foraging environment most of the time.
In Adams county Pennsylvania in April, apples are in need of pollinators. Contiguous acres of apple orchards are all blooming, yet colonies placed in orchards with 5 acre blocks in full bloom collect anywhere from 2-70% of their total pollen from these apples on any given day. The pesticide history in this orchard is thus not a very good predictor of the potential pollen pesticide residues that are likely to wind up in the colonies placed there for pollination. Thus, commercial beekeepers and local beekeepers alike may be surprised by the mixed sources of pollen in their colonies and the ranges from which they have been gathered. This has important implications for the pesticide residues to be found in any given colony at any given time. Having a landscape level appreciation for the foraging range of your colonies will help give you a realistic perspective on potential sources of pesticide contamination. Drive around your area and take a look at the possible places where pesticides may be used and talk to the people involved.
- Communicate with individuals/facilities that are likely users of pesticides in your foraging area and encourage them not to spray insecticides or fungicides during bloom on any plants.
- Encourage growers of bee pollinated crops to plant buffers of blooming plants for pollinators and home owners to “plant for pollinators”
- Replace combs often to prevent pesticide build-up.
- If you have a pesticide poisoning incident report it. Verified pesticide levels in your dead bees make this a much stronger incident report.
Pesticides with known toxicity to honey bees have a warning on the label that must be followed by anyone who applies the material. “ This product is highly toxic to bees exposed to direct treatment or residues on blooming crops or weeds. Do not apply this product or allow it to drift to blooming crops if bees are visiting the treatment area.” While these are explicit warnings, it is far too easy to ignore the presence of honey bees and especially native species of bees when spraying during bloom. The important point is that if pesticides are applied anytime during bloom, bees will be killed; even the shortest duration pesticides like pyrethroids are not disappearing overnight, so spraying late one day does not guarantee that bees will not receive a toxic dose the following days. Not following these restrictions constitutes not following the law, and bee kills resulting from such uses should be reported as a bee kill incident. To do this, each beekeeper must be vigilant about the health of their colonies and if pesticide exposure and resulting bee kills are suspected, then an immediate response is best. Collect bees and keep them in a freezer until they can be sent for analysis. We have been able to support a beekeeper cost share program to help offset the high costs of pesticide analyses, so samples may be submitted to us by contacting Maryann Frazier at mfrazier@psu.edu. Bee incidents should also be reported to your state department of agriculture or regulatory authority overseeing pesticide use in your state, as well as to the manufacturer of the pesticide involved. Along with contacting these agencies, we would also recommend that you make an incident report to the Environmental Protection Agency by visiting their website and completing the form as completely as you can. Without such reporting, the regulatory agencies have no information to indicate that anything is of concern about current pesticides or the manner in which they are being used or misused. The experiences of our migratory beekeepers indicates that pesticide misuse is a widespread occurrence and incident reporting is the best method of countering these actions.

Figure 4. The dynamic foraging of a typical honey bee colony includes a range of 3.73 miles radius 95% of the time, with a range up to 6.21 miles in times of limited rsources, with teh amility to detect the maximum rewarding nectar within a two-hour period (Modified from Seeley, 1995).
References
- Agnello, A., A. Atanassov, J. C. Bergh, D. J. Biddinger, L. J. Gut, M. J. Haas, J. K. Harper, H. W. Hogmire, L. A. Hull, L. F. Kime, G. Krawczyk, P. S. McGhee, J. P. Nyrop, W. H. Reissig, P. W. Shearer, R. W. Straub, R. T. Villanueva, and J. F. Walgenbach. 2009. Reduced-risk pest management programs for eastern U.S. apple and peach orchards: a 4–year regional project. American Entomologist 55(3): 184-197.
- Atkins, E. L., D. Kellum, and K. W. Atkins. 1981. Reducing pesticide hazards to honey bees: Mortality prediction and integrated management strategies. Univ. Calif. Div. Agric. Sci. Leafl. 2883.
- Chauzat, M-P. A-C. Martel, C. Gougoule, P. Porta, J. Lachaize, S. Zeggane,M. Aupert, P. Carpentierand J-P. Faucon. An Assessment of Honey bee Colony Matgricdes, Apis mellifera Hymenoptera:Apidae) to Monitor Pesticide Presence in Continental France. Environental Toxicology and Chemistry, Vol. 30, No. 1, pp. 103–111.
- Cox, R. L., and W. T. Wilson. 1984. Effects of permethrin on the behavior of individually tagged honey bees, Apis mellifera L (Hymenoptera, Apidae). Environ. Entomol. 13: 375-378.
- Decourtye, A., C. Armengaud, M. Ranou, J. Devillers, S. Cluzeau, M. Gauthier, M. Pham-Delegue. 2004. Imidacloprid impairs memory and brain metalbolism in the honeybee (Apis mellifera L.). Pestic. Biochem. Physiol. 78:83-92.
- Decourtye A, Lefort S, Devillers J, Gauthier M, Aupinel P, Tisseur M. 2009. Sublethal effects of fipronil on the ability of honeybees (Apis mellifera L.) to orientate in a complex maze, Julius-Kühn-Archiv; 423:75-83.
- Decourtye A, Devillers J, Aupinel P, Brun F, Bagnis C, Fourrier J, Gauthier M. 2011. Honeybee tracking with microchips: a new methodology to measure the effects of pesticides. Ecotoxicology; 20:429-437.
- Desneux, N., A. Decourtye, and J.-M. Delpuech. 2007. The sublethal effects of pesticides on beneficial Arthropods. Annu. Rev. Entomol. 52:81-106.
- El Hassani AK, Dacher M, Gary V, et al. 2008. Effects of sublethal doses of acetamiprid and thiamethoxam on the behavior of the honeybee (Apis mellifera). Arch Environ Contam Toxicol; 54:653-661.
- EPA-OPP. 2005. Reregistration eligibility decision for tau-fluvalinate. Docket 20050230
- Frazier, M., C. Mullin, J. Frazier, and S. Ashcraft. 2008. What have pesticides got to do with it? American Bee Journal 148: 521-523.
- Girolami V, Mazzon L, Sqartini A, Mori N, Marzaro M, Di Bernardo A, Greatti M, Giorio C, Tapparo A. 2009. Translocation of Neonicotinoid Insecticides From Coated Seeds to Seedling Guttation Drops: A Novel Way of Intoxication for Bees. Journal of Economic Entomology.
- 102: 1808-1815.
- Gradish A.E., (2009) Non-target effects of reduced risk pesticides on beneficial arthropods used in greenhouse vegetable production MSc Thesis University of Guelph.
- Grube, A. D. Donaldson, T. Kiely and L. Wu. 2011. Pesticide Industry Sales and Usage. 2006 and 2007 Market Estimates. US EPA. Report 31 pp.
- Halm, M. P., A. Rortais, G. Arnold, J. N. Tasei, and S. Rault. 2006. New risk assessment approach for systemic insecticides: The case of honey bees and imidacloprid (Gaucho). Environ. Sci. Technol. 40: 2448-2454.
- Huntzinger, C.I., James, R.R., Bosch, J., and Kemp, W.P. (2008) Fungicide tests on adult alfalfa leafcutter bees (Hymenoptera: Megachilidae) J Econ Entomol 101 (4) 1088-1094.
- Iwasa, T., N. Motoyama, J. T. Ambrose, and R. M. Roe. 2004. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot. 23:371-378.
- Ministerium Für Ernährung Und Lädlichen Raum Baden-Württemberg. Abschlussbericht Beizung und Bienenschäden 2008. Internet: http://www.mlr.baden-wuerttemberg.de/mlr/allgemein/Abschlussbericht_Bien…
- Mullin, C. A., M. Frazier, J. L. Frazier, S. Ashcraft, R. Simonds, D. vanEngelsdorp, and J. S. Pettis. 2010. High Levels of Miticides and Agrochemicals in North American Apiaries: Implications for Honey Bee Health. Plos One 5: e9754.
- Nguyen, B.K., Mignon, J., Laget, D., de Graaf, D.D., Jacobs, F.J., van Engelsdorp, D., Brostaux, Y., Saegerman, C., Haubruge, E., 2010. Honey bee colony losses in Belgium during the 2008–9 winter. J. Apic. Res. 49, 337–339.
- Oliver, R. 2010. Sick Bees – Part 2: A Model of Colony Collapse. American Bee Journal September: Vol. 150, No. 9, 865-872.
- Pilling, E. D., and P. C. Jepson. 1993. Synergism between EBI fungicides and a pyrethroid insecticide in the honeybee (Apis mellifera). Pestic. Sci. 39: 293-297.
- Saegerman, C., N. Speybroeck, S. Roels, E. Vanopdenbosch,E. Thiry, and D. Berkvens. 2004. Decision support tools for clinical diagnosis of disease in cows with suspected bovine spongiform encephalopathy. J. Clin. Microbiol. 42: 172-178.
- Scott-Dupree, C.D., Conrol, L., Harris, C.R., 2009. Impact of currently used or potentially useful insecticides for canola agroe-cosystems on Bombus impatiens, (Hymenoptera: Apidae), Megachile rotundata (Hymenoptera: Megachilidae) and Osmia lignaria (Hymenoptera: Megachilidae). J Econ Entomol 102 (1) 177-182.
- Seeley, T. 1995. The Wisdom of the Hive. Harvard University Press. Cambridge.
- Suchail, S., G. De Sousa, R. Rahmani, and L. P. Belzunces. 2004. In vivo distribution and metabolisation of C-14-imidacloprid in different compartments of Apis mellifera L. Pestic. Mgt. Sci. 60: 10561062.
- Tennekes, H.A., 2010. The significance of the Druckrey–Küpfmüller equation for risk assess-ment—the toxicity of neonicotinoid insecticides to arthropods is reinforced by exposure time. Toxicology 276, 1–4.
- Thompson HM, Wilkins S, Batterby AH, Waite RJ, Wilkinson D (2007). Modelling long-term effects of IGRs on honey bee colonies. Pest Manag Sci 63: 1081–1084.
- Vandame, R., M. Meled, M. E. Colin, and L. P. Belzunces. 1995. Alteration of the homing-flight in the honey bee Apis mellifera L. exposed to sublethal dose of deltamethrin. Environ. Toxicol. Chem. 14: 855-860.
- vanEnglesdorp, D. N. Speybock, J.D. Evans, B.K. Nguyen, C. Mullin, J. Frazier, M. Frazier, D. Cox-Foster, Y. Chen, D. Y. Tarpy, D. Haubruge, J.S. Pettis, and C, Sagermann. 2010. Weighing Risk. Factors Associated With Bee Colony Collapse Disorder by Classification and Regression Tree Analysis. J Econ. Entomol. 103(5): 1517-1523.
- Wallner, K. 2009. Guttation: Tropfen, die es in sich haben. Deutsches Bienen-Journal 4: 18-19.
- vanEngelsdorp D, Evans JD, Saegerman C, Mullin C, Haubruge E, et al. 2009. Colony Collapse Disorder: A Descriptive Study. PLoS ONE 4(8): e6481.
- Wu JY, Anelli CM, Sheppard WS .2011 Sub-Lethal Effects of Pesticide Residues in Brood Comb on Worker Honey Bee (Apis mellifera) Development and Longevity. PLoS ONE 6(2): e14720.
