- Published in the American Bee Journal, June 2010
Download a .pdf of the 2010 ABRC proceedings.
The 2010 American Bee Research Conference was held January 14-15 at the Wyndham Orlando Resort in Orlando, Florida. This was a special joint conference between the American Association of Professional Apiculturists and the Canadian Association of Professional Apiculturists. The twenty fourth American Bee Research Conference will be held in conjunction with a joint meeting of the American Beekeeping Federation and the American Honey Producers Association in Galveston, TX on January 4-8, 2011. The following are abstracts from the 2010 Conference.
Video recordings of selected presentations
- ABRC2010 A New Assay to Measure Mite Grooming Behavior
- ABRC2010 Pesticide in Pollen Trapped from Honey Bee Hives in Connecticut
- ABRC2010 A Test for Sub-acute Effects of Some Commonly Used Bee Hive Chemicals
- ABRC2010 Pests Pathogens and Queen Losses in Commercial Migratory Operations
- ABRC2010 Apis mellifera Proteomics of Innate Resistance
- ABRC2010 Variability and Correlations Among Five Traits Associated with American Foulbrood Resistance in a Canadian Breeding Population
- ABRC2010 Effects of a Miticide on Honeybee Memory – is the Cure Worse than the Disease
- ABRC2010 Nosema ceranae in North America Nasty Nice or Neither
- ABRC2010 Beneficial Microflora in Honey Bee Colonies
- ABRC2010 Molecular Diagnostics of Nosema ceranae and apis from Honey Bees in New York
- ABRC2010 Nestmate Recognition in the Honey Bee Apis mellifera
- ABRC2010 Effects of Varroa Mites and Bee Diseases on Pollination Efficacy of Honey Bees
- ABRC2010 Effect of Transportation on Honey Bee Physiology
- ABRC2010 Disinfection of Nosema ceranae Infected Comb by Irradiation Acetic Acid and Heat
- ABRC2010 Interactions Between Nosema Microspores and a Neonicotinoid on Honey Bees
- ABRC2010 Pesticide Analysis at the Stationary Apiaries
- ABRC2010 Bee Health at eXtension
- ABRC2010 Drug Interactions Between In-hive Miticides and Fungicides in Honey Bees
- ABRC2010 Bee CAP Overview and Stationary Apiaries
- ABRC2010 Preference of Phoretic Mites for Young Bees Increase Varroa Fitness
- Varroa Sensitive Hygiene and Mite Reproduction
- ABRC2010 Safe Exchange of Honey Bee Germplasm
Presentation abstracts
1. Afik, O., W.B. Hunter & K.S. Delaplane – EFFECTS OF VARROA MITES AND BEE DISEASES ON POLLINATION EFFICACY OF HONEY BEES – WATCH VIDEO – Varroa mites and viral diseases are known to affect the efficiency of crop pollination by honey bees through the elimination of colonies, but only limited information exists on their influence on pollination at sub-lethal levels on the individual bee (Ellis & Delaplane, 2008 Agr. Ecosyst. Environ. 127:201-206). The purpose of this study was to learn about effects that varroa mites and bee diseases may be having on the foraging behavior of adult bees and the consequences of these effects on successful fruit pollination. For the first season of the experiment, four honey bee colonies of about 4,500 bees each were established. Two of these colonies were each infested with 1,000 varroa mites collected from other hives by sugar powdering. Two other colonies were used as non-infested control colonies. In order to force mites to attach to the adult bees, brood combs from both treatments were replaced with empty combs before brood was sealed. Each colony was caged in a separate enclosure containing one blueberry target plant and two potted pollen source plants. Pollination efficacy was tested by measuring percent of fruit-set and pollen deposition at flowers exposed to a single visit by an individual bee. Each visiting bee was collected at the end of the flower visit and preserved for later pathogen analysis.
The results indicated that bees from mite-infested colonies achieved a lower percent of fruit set and tended to deposit fewer pollen grains on the flower stigma. Bees from infested colonies performed shorter flower visits and a lower percentage of them were pollen foragers. These two behavioral differences may contribute to lower rate of fruit-set since the duration of flower visit was positively correlated with pollen deposition and pollen foragers were found to be more efficient pollinators of blueberry flowers than nectar foragers. More than 75% of the bees from both treatments were determined to be naturally infected with the viruses DWV and BQCV, but no bee was positive for Nosema spp., ABPV, IAPV or KBV. The results suggest that bees from colonies highly infested with mites are less efficient pollinators, possibly due to shorter visits to the flowers and lower tendency to collect pollen. The effects of mite infestation combined with high virus infections have not yet been determined. Further research will focus on how to limit the effects of varroa mites on the foraging behavior and pollination success of honey bees.
2. Alaux, C., J.-L. Brunet, C. Dussaubat, F. Mondetd, S. Tchamitchand, M. Cousind, J. Brillard, A. Baldy, L.P. Belzunces & Y. Le Conte – INTERACTIONS BETWEEN NOSEMA MICROSPORES AND A NEONICOTINOID IN HONEY BEES Watch Video – Massive honey bee losses have been reported in the world, but the specific causes are still unknown. Single factors, like pesticide impact, or a disease or parasite have not explained this global decline, leading to the hypothesis of a multifactorial syndrome (van Engelsdorp et al., 2009 PLoS One 4:e6481). Consequently, we tested the integrative effects of an infectious organism (Nosema sp) and an insecticide (imidacloprid) on honeybee health. We demonstrated, for the first time, that a synergistic effect between both agents, at concentrations encountered in nature, significantly weakened honey bees. The combination of Nosema, a pathogen whose importance is emerging, with imidacloprid caused a significantly higher rate of individual mortality and energetic stress in the short term than either agent alone. We then quantified the strength of immunity of honey bees. While the single or combined treatments showed no effect on individual immunity (haemocyte number and phenoloxidase activity), a measure of colony level immunity, glucose oxidase activity, was significantly decreased only by the combined treatments, emphasizing their synergistic effects. Glucose oxidase activity enables bees to secrete antiseptics in honey and brood food. This suggests a higher susceptibility of the hive to pathogens. We, thus, provide evidence for integrative effects of different agents on honey bee health, both in the short and long term. By focusing either on the effects of pesticides or parasites alone, previously established synergy has been ignored, despite clear evidence from integrated pest management that entomogenous fungi act synergistically with sub-lethal doses of pesticides to kill insect pests (Alaux et al., 2009 Environ. Microb. doi:10.1111/j.1462-2920.2009.02123.x).
3. Andinof, G.K. & G.J. Hunt – A NEW ASSAY TO MEASURE MITE GROOMING BEHAVIOR – Grooming behavior is one of the known mechanisms of defense for honey bees against parasitic mites. Varroa destructor is often considered the biggest beekeeping problem within the U.S. and around the world. Mite-grooming behavior has been described as the ability of the adult bees to remove Varroa mites during grooming and has been associated with mites that have been chewed by the bees’ mandibles, but the proportion of chewed mites is extremely tedious to measure.
We developed an easier assay to measure mite-grooming behavior that can be used for selection in breeding programs. Wood cages with screened tops and bottoms were used to hold a frame of bees collected from the brood nest. Bees were transferred to comb containing pollen and nectar but without brood. The mites removed during grooming were collected in sticky boards for three days at room temperature (22-25 °C) and then counted. The remaining mites on the adult bees were collected and counted using carbon dioxide (CO2) to anesthetize the bees and powdered sugar to remove the mites. The percentage of the mites removed was calculated.
A significant relationship (p = 0.0285) was found between the proportion of mites removed in the lab assay and the proportion of chewed mites on sticky boards from the source colonies. This relationship indicates that the colonies that removed the highest percentage of mites in the caged adult bees were also the colonies that had the highest percentage of chewed mites (Figure). These results suggest that the method used to measure mite-grooming behavior is effective. In addition, we also found a negative relationship (p = 0.0072) between the percentage of mites removed and mite infestation of adult bees, which indicates that the colonies with the highest percentage of mites removed in the cage assay, had the lowest population of mites on adult bees. These results suggest that the low population of mites present on the adult bees is due to grooming.
4. Bahrein, R. & R.W. Currie – INCREASING THE ECONOMIC THRESHOLD FOR FALL TREATMENT OF VARROA MITE (VARROA DESTRUCTOR A.&T.) IN HONEY BEES BY USING MITE-TOLERANT STOCKS IN NORTHERN CLIMATES – The objective of this research was to develop effective and economical methods to reduce the impact of varroa mites on honey bees under winter management systems. Fall economic thresholds for varroa mite control in the prairie region of Canada suggest producers should treat honey bee stock when the mite level is greater than 4 mites per 100 bees (in late August to early September) to prevent fall or winter colony loss (Currie & Gatien, 2006 Can. Entomol. 138:238-252). However, it is not known how the use of mite tolerant stock or late season acaricide application would affect these thresholds. An experiment to assess these factors was carried out at University of Manitoba in fall 2007 to spring 2008. Thirty nine colonies from mite-susceptible (n=23) and mite-tolerant (n=16) stocks with mite levels (16±3 mites per 100 bees) above the fall economic threshold were chosen and within each type of stock were randomly assigned into two groups that would either receive a late fall (November 2007) treatment with 1 g of oxalic acid crystals or were left untreated. Colonies were randomly arranged in two small rooms in a wintering building maintained at 5°C. Colony worker population and mean abundance of varroa mites were assessed before and after wintering colonies, and varroa mite and worker mortality rates were determined.
As expected, late fall treatment with oxalic acid reduced the mean abundance of varroa mites over winter (to 3.5%), relative to that found in untreated colonies (12%) in both susceptible and tolerant stock as indicated by a significant acaricide treatment × season interaction (P<0.01). However, under high fall mite load, reductions in mite levels associated with late-season oxalic acid treatment did not improve colony survival relative to untreated colonies. The use of mite tolerant stock improved colony survival. In the mite-tolerant stock winter survival of colonies was much higher (75%) than in mite-susceptible stock (43%). The populations of worker bees in mite-tolerant and mite-susceptible stock were similar in colonies that survived winter. Bee populations in tolerant stock tended to be slightly higher than in susceptible stock whether colonies were treated with acaricide or not. Untreated colonies with tolerant and susceptible stocks had similar mite mortality rates over winter but tolerant stock had slightly a lower mean abundance of mites at the end of winter compared to susceptible stock. Overall this study demonstrate that when late fall mite levels are well above the fall economic threshold, tolerant stock could be used by beekeepers to help minimize colony loss in the Canadian prairies and under these conditions late fall oxalic treatments may not improve colony survival.
5. Cobey, S., J. Pollard, C. Plante, M. Flenniken & W.S. Sheppard – DEVELOPMENT OF A PROTOCOL FOR THE INTERNATIONAL EXCHANGE OF HONEY BEE GERMPLASM Watch Video- The development of protocol for the safe, well regulated international exchange of honey bee genetics is needed. The current ban on importation is inconsistent and has failed to prevent the spread of pests, parasites and pathogens. The initial limited gene pool introduced into the U.S. before the 1922 ban and the alarmingly high loss of colonies due to Colony Collapse Disorder is an increasing concern. Genetic diversity has been demonstrated to increase colony fitness and reduce the impact of pests and diseases. Our project is designed to develop technologies to safely import honey bee germplasm, semen and eggs, and to import stocks selected for resistance to enhance our domestic honey bee gene pool.
An improved bee semen extender with an antibiotic mixture, containing gentamicin, amoxicillin, lincomycin and tylosin, specifically designed to control bacterial pathogens was developed and tested to facilitate the transport of semen. Extended semen was examined for viability and motility after storage for 7 days, and inseminated to virgin queens. Results demonstrated high sperm viability, normal spermathecal sperm counts and normal brood patterns of inseminated queens. USDA-APHIS (Animal Plant Health Inspection Service) permits were obtained and honey bee semen imported. Apis mellifera ligustica from survivor stock in Italy and A. m. carnica from the Germany Carnica Association were imported in 2008 and 2009 and crossed with domestic stocks. The semen was tested for viruses and resulting colonies established in an approved quarantine area at Washington State University. Progeny of these colonies were also examined and tested for pathogens. The 2008 imports released were backcrossed to the 2009 imports to create more pure stocks and also were incorporated into proven commercial U.S. stocks.
The New World Carniolan × German A.m. carnica colonies expressed increased fitness and increased expression of hygienic behavior. The Italian stock is still undergoing testing. Future plans are to import A.m. caucasica, as this subspecies is detectable but largely unrecognizable in the U.S.
Honey bee eggs represent a complete genetic package and are available in large quantities. Therefore, we developed reproductive technologies to manipulate honey bee eggs to allow for their isolation, pathogen testing and transport. A method to manipulate embryos was developed using fine forceps modified by the application of micro-bore tubing. The transferred eggs were hatched in vitro and the larva were grafted into queen cell cups, reared into queens and instrumentally inseminated with a high rate of success.
6. Delaplane, K.S. & J.A. Berry – A TEST FOR SUB-LETHAL EFFECTS OF SOME COMMONLY USED HIVE CHEMICALS, YEAR TWO WATCH VIDEO – We are involved in a two-year, two-state (GA, SC) experiment examining sub-lethal effects of selected bee hive chemicals; the list includes registered products at label rates as well as two off-label formulations. The reason we are doing this is that there is evidence that some of the chemicals used in beekeeping are hazardous to bees and contribute to bee decline (Frazier et al., 2008 Am. Bee J. 148(6):521-523; Desneux et al., 2007 Ann. Rev. Entomol. 52:81-106). Understanding this piece of the CCD puzzle will help beekeepers move toward more chemical-independent management. Here are results for two years from Georgia. Varroa levels (mites / 100 bees) were significantly higher in CheckMite (coumaphos)-treated colonies than in colonies treated with Taktic (amitraz); mite levels were intermediate in all other treatments. Bees in the non-treated control colonies exhibited numerically highest brood viability, homing ability, and foraging rate and numerically lowest incidence of queen supersedure cells. Information like this is important for evaluating the cost : benefit ratio of using exotic chemicals in honey bee management.
Table – Comparison of sublethal effects of various chemicals commonly used in hives.
|
Chemical |
Mites per 100 adult bees |
Brood viability (% open brood alive after 3 d) |
Supersedure cells |
Homing ability *1 |
Foraging rate *2 |
|
Non-treated |
11±2 (22)ab |
96.6±0.9(33) |
0.5±0.3(35) |
17±2.5(8) |
57±9(32) |
|
Cu naphthenate *3 |
13±2(19)ab |
89.2±2.7(30) |
2.2±0.7(32) |
10.4±2(7) |
54±8(29) |
|
Apistan (fluvalinate) |
7±1.4(21)ab |
92.8±1.4(31) |
3±1(33) |
16±2(6) |
52±8(30) |
|
CheckMite (coumaphos) |
14±2.6(20)a |
90.1±2.4(30) |
3.3±1(32) |
9.5±2(6) |
51±7(29) |
|
Maverik (fluvalinate) |
5±1.3(19)ab |
90.5±2.2(29) |
1.4±0.6(30) |
13.9±3(7) |
53±7(29) |
|
Taktic (amitraz) |
3±1(22)b |
91.3±2.1(29) |
2.1±0.5(34) |
15±7(7) |
50±7(31) |
- Reported as % of bees (of 30 marked) returning to colony entrance within 15 min after being released 1 km distant.
- Reported as number of foraging bees returning to colony entrance per min in observation period of 15 min.
- Copper naphthenate wood preservative was applied as a 2% water-soluble solution on a plywood panel which was allowed to air-dry and placed on hive floor.
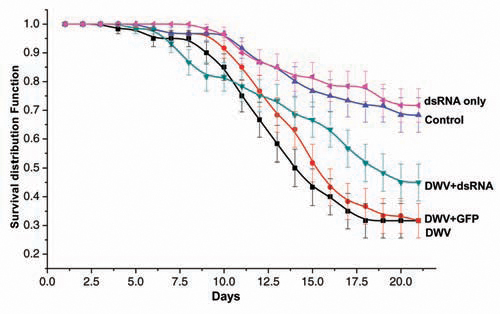
7. Desai, S. & R.W. Currie – INHIBITION OF DEFORMED WING VIRUS (DWV) MULTIPLICATION IN HONEY BEES BY RNA INTERFERENCE – DWV plays a major role in affecting honey bee health. High proportions of colonies are infected by this virus, and it can be detected in worker honey bees, queens, pupae, larvae, drones and also in varroa mites. DWV and its interactions with the ectoparasitic varroa mite and other diseases have caused significant mortality of honey bee colonies on a world-wide basis (Miranda & Genersch, 2009 J. Invertbre. Pathol.103:S48-S61). RNAi is a comparatively “simple”, rapid and specific method for silencing gene function and can be developed to be specific to an individual virus. RNAi has recently been utilized in a number of species including human beings, plants, animals and insects (Drosophila) and recently in bees to suppress viruses. For example, successful silencing of Israeli Acute Paralysis Virus (IAPV) in honey bees by feeding specific dsRNA to bees dramatically improved bee-to-brood ratio and honey yield (Maori et al., 2009 Insect Mol. Biol. 18:55-60).
RNAi reduces virus replication by causing degradation of the target mRNA. In this experiment, we assessed the effects of feeding dsRNA constructs against DWV to larvae that were infected with DWV and the potential lethal and sub-lethal effects on developing worker bees.
In DWV-infected larvae fed dsRNA survival (45%) was greater than the survival of larvae fed unrelated dsRNA (GFP) (31%) or DWV-infected larvae that were not treated with dsRNA. The dsRNA did not affect larval survival as DWV-“free” larvae fed our dsRNA construct had similar survival to that of untreated controls (Figure). Our dsRNA-fed larvae that were infected with DWV had significantly lower levels of wing deformity compared to larvae infected DWV or to larvae infected with DWV and an unspecific form of RNAi (GFP). Our experiment also demonstrated for the first time that feeding DWV orally in the absence of mites causes wing deformity in in-vitro reared larvae. We hypothesize that application of dsRNA into the honeybees fed DWV should result in a reduction in DWV titer over time with no effect on bee longevity. If proven effective, this mechanism can be used to block DWV and could improve winter survival of honeybee colonies.
8. Eischen, F.A., R.H. Graham & R. Rivera – MOUNTAINSIDE WINTERING IMPROVES COLONY STRENGTH AND SURVIVAL OF HONEY BEES IN SOUTHERN CALIFORNIA – We examined the interaction of a feeding program and cold-windy conditions on honey bee colonies near Santa Ysabel, California (elev. 914 m). An equal number of colonies located near Fallbrook, California (elev. 219 m) served as controls. The trial began 7 September 2008 near Holtville, California (Imperial Valley). Colonies were randomly assigned to four treatment groups (n = 50), i.e., 1) Highland, fed continuously, 2) Highland, fed discontinuously, 3) Lowland, fed continuously, and 4) Lowland, fed discontinuously. On 20 November, lowland-designated colonies were moved to their normal winter locations near Valley Center, CA, and highland colonies to a mountainside near Santa Ysabel, CA. Groups 1) and 3) were fed continuously throughout the trial. Groups 2) and 4) were not fed during the period 6 Dec. 2008 – 13 Jan. 2009. Colonies were evaluated for strength and broodnest size on 26 January 2009, i.e., near the time of almond pollination evaluation. Regardless of feeding treatment, highland colonies at the end of the trial were stronger by about 1.5 frames of bees than colonies of either lowland group. Brood nests of highland colonies were smaller, however by about 1.0 frames of brood. Stored pollen declined in the highland colonies, but stayed about the same in the lowland colonies; indicating that pollen foraging occurred in the lowland colonies. Highland colonies had a slight, but significantly higher survival rate than did lowland colonies.
To determine if the highland colonies would lose strength on return to lowland conditions, colonies from each treatment group (n = 25) were moved to an almond orchard near Shafter, CA and examined on February 15. Highland colonies were nominally larger than lowland colonies. Broodnest sizes were about the same for both highland and lowland colonies. Highland colonies had significantly more stored pollen than lowland colonies, indicating that their larger size caused increased pollen foraging. A simplified cost/benefit analysis indicates that it was economical to place colonies in a climate that limits unproductive flight during winter.
9. Eischen, F.A., R.H. Graham & R. Rivera – ALMOND POLLEN COLLECTION BY HONEY BEE COLONIES HEAVILY INFECTED WITH NOSEMA CERANAE – In 2007 apiculturists became aware that the microsporidian, Nosema ceranae, had become established in the United States. A related species, N. apis is a well known honey bee pathogen. There was concern within the beekeeping industry that this “new” pathogen is part of the Colony Collapse Disorder (CCD) phenomenon.
A commercial beekeeper, based in Louisiana and New York was found to have high levels of this pathogen in colonies used to pollinate almonds, blueberries and cranberries. We examined the impact of four N. ceranae levels on honey bee colonies including pollen collection during almond bloom in the Central Valley of California during February – March 2009.
N. ceranae levels in October 2008 were on average 1.0 – 2.9 million spores/bee (MSPB). By January 2009, levels increased to, on average, 1.6 MSPB in the lightest infection group to 49.5 MSPB in the heaviest. After transport from Louisiana to California during 31 Jan.-2 Feb, colonies in the two heaviest-infected groups had striking declines in their spore levels. We suspect the rigors of travel caused many severely infected bees to die.
Pollen collection by the lightest-infected colonies (Group I) was about twice that of Group II (159.8 vs. 74.0 g/day). Both Group I (0-4.5 MSPB) and Group II (5-15 MSPB) colonies collected significantly more pollen than Groups III (16-34 MSPB) and IV (35-49 MSPB) 16-34. When pollen collection was based on grams of pollen per frame of adult bees, we found that Group I colonies collected significantly more pollen. This suggests that foragers with heavy infections either make fewer collecting trips or pack smaller loads or both.
Colonies of all four groups lost significant adult bee strength during almond bloom, but loses were more severe in Groups II, III, and IV. At the end of pollination, no significant differences in N. ceranae spore levels were found among treatment groups, but levels rose in Groups I and II, while remaining about the same in Groups III and IV.
We suspect that these colonies, especially those with high spore levels had large spore reservoirs on their honeycombs. We recommend including this factor when determining economic thresholds.
10. Eitzer, B., F. Drummond, J.D. Ellis, N. Ostiguy, M. Spivak, K. Aronstein, W.S. Sheppard, K. Visscher, D. Cox-Foster & A. Averill – PESTICIDE ANALYSIS AT THE STATIONARY APIARIES Watch Video- One facet of the stationary apiary project within the “Sustainable Solutions to Problems Affecting the Health of Managed Bees Coordinated Agricultural Program” is a monitoring of the honey bee’s exposure to pesticides. This is being done by determining pesticide residues in the pollen that is brought back to the hive by foraging honey bees. At five hives from each of the stationary apiaries, pollen is sampled with traps one day per week. Pollen samples are frozen after collection. Aliquots from all samples taken from an apiary during a calendar month are combined to generate a monthly composite sample for each apiary. Five grams of this composite sample are analyzed by a multi-pesticide residue procedure. In brief, the samples are extracted with acetonitrile using a dispersive solid phase technique known as QuEChERS (for Quick, Easy, Cheap, Effective, Rugged and Safe) and analyzed using high performance liquid chromatography/mass spectrometry/mass spectrometry. Using this technique allows over 140 different pesticides to be analyzed in the parts per billion (PPB) concentration range.
To date 29 of the monthly composite samples have been analyzed. Within these 29 samples, residues of 32 different pesticides or pesticide metabolites have been observed including: 14 insecticides plus one insecticide metabolite, 9 fungicides and 8 herbicides. The average composite pollen sample had an average of 4.1 pesticide residues detected. The concentration of residues when detected are mostly in the low PPB range (1< to 30 ppb) but some residues were substantially higher. The results indicate that honey bees at the stationary apiaries are being exposed to varying amounts of pesticides. As might be expected, this exposure amount varies with the location of the apiary (i.e. honey bees in Washington are exposed to different pesticides than those in Florida) and time of year. In addition, analysis of non-composited samples taken from five different hives within the same apiary on the same day also shows different pesticide amounts. This indicates that the honey bees from these hives are clearly foraging from different fields that have had different amounts of pesticides applied. This variability of pesticide exposure will be further examined as we continue to monitor these hives over the next several years.
11. Esaias, W. – RELATIONSHIPS BETWEEN VEGETATION COVER, NECTAR AVAILABILITY, AND THE AFRICANIZED HONEY BEE – Collections of scale hive records of the Honey Bee Nectar Flow reveal dramatic regional variations related to honey bee forage and its phenology, and are used to quantify inter-annual variations that are related to changes in land cover type (nectar sources) and natural climate change. Temporal trends in the nectar flow dates correlate well with trends in vegetation parameters observed with the Moderate Resolution Imaging Spectroradiometer on the Terra and Aqua satellites. Nectar flows are generally occurring earlier in the Northeast U.S., and later in the Southeast U.S., in conjunction with regional increases in winter minimum temperatures. Numbers of volunteer beekeepers who provide records of daily weight changes has been doubling for the past several years and is now approaching 100 locations throughout the U.S. Further insight into climate and land cover change impacts on the timing of nectar flows will be possible as the number of volunteer locations increases, especially in the central and western U.S. Maps of site locations coverage, and scale hive data itself, are available at http://honeybeenet.gsfc.nasa.gov. Research programs establishing longer term monitoring apiaries are encouraged to consider monitoring hive weight changes to evaluate the impact of inter-annual nectar flow variations on colony health and behavior.
Jointly with the USGS National Institute of Invasive Species at Ft. Collins (C. Jarnevich, J. Morisette, T. Stohgren), climate and satellite vegetation data and species distribution models (SDMs) are used to better understand the areas at risk from further advance of the Africanized Honey Bee, and to shed light on why its movement into eastern Gulf of Mexico state has been slow compared to movement to the north and west. A key limitation to these studies, based on presence of an invasion still in progress, is the relatively poor knowledge of exact AHB locations throughout the range, although some states are very well sampled. Additionally, the sampling is biased spatially, makes no distinction between overwintered versus incidental/transient transport, and sampling effort is not uniform or recurrent over time. With 1-5 km scale resolution, model depictions of areas having similar climate and vegetation to the presence locations appear to be very robust in the Southwest U.S. (west of 190 W) using the Maxent model. Winter and summer temperatures and vegetation parameters were critical variables. Maxent does not give satisfactory results for the Southeast U.S. yet. There, sampling biases are extreme due to presence data only in the western portion and extreme southeastern (S. FL) portion of the region. However, initial software test runs using an ensemble approach with 5 different SDMs appear to provide very useful maps of suitable AHB regions for the U.S., with further refinement required. Based on those very preliminary results and the small number of historic and current nectar flow records available, there is complete correspondence between areas of AHB presence/absence and abundance/dearth of nectar in the late summer and fall. This suggests that the combination of physical climate and the bulk vegetation phenology data from satellite observations can provide useful insight into local nectar flow phenology, at national scales.
Contributors to this project are R. Wolfe, P. Ma, J. Nightingale, and J. Nickeson at GSFC, C. Jarnevich, T. Stohlgren, J. Morisette at USGS Ft. Collins, J. Pettis at ARS/USDA Beltsville, J. Harrison at Arizona State Univ, J. Hayes at FL DACS, D. Downey at UT DAF, and the HoneyBeeNet Volunteers. Funding is from the NASA Earth Sciences Applications – Decisions Program.
12. Fell, R., C. Brewster, & A. Mullins – THE SPATIAL DISTRIBUTION OF VARROA MITES IN HONEY BEE HIVES – Studies on the intra-hive distribution of Varroa mites were designed to obtain a better understanding of the spatial distribution of mites, how these patterns change over time, and how this information might be used to improve sampling and treatment decisions. Mite populations were sampled in a group of eight experimental hives (consisting of 1 full-depth hive body or 1 full-depth and 1 medium depth) three times at two-week intervals from mid-August to early October. PSU/IPM sticky boards were used for sampling but were modified to cover the entire bottom board of a hive. Sticky boards were left in hives for 3 days. After removal, mite numbers were counted in each grid square (1.8 x 1.8 cm) and used to establish a distribution matrix. A geostatistical approach utilizing GS+ and Matlab (MathWorks Inc., Natick, MA) software was used to analyze the mite sampling data and to build spatial models of mite distributions that can be displayed as surface density maps (Figure). Brood distribution in each hive was also measured after mite sampling using digital images. Frames were removed and photographed on each side with respect to their position in the hive and then divided in to a set of data cells that corresponded with the sticky board grid. Frame contents were categorized as brood (worker, drone, capped, uncapped) or non-brood. Mite and brood sample distributions were further analyzed using spatial analysis by distance indices (SADIE).
The results show mite distributions were aggregated or clumped, and significantly associated with brood distributions (Index of association [Im] values varied from 0.23 – 0.58, Pm < 0.0001). Surface density maps indicate that bee collection for mite sampling using techniques such as the powdered sugar roll should be made in or near the brood nest. The results of this study also indicate that mite-sampling data can be highly variable. Mite numbers from sticky board samples were found to vary by as much as 250% in as little as two weeks. These data make it difficult to set mite number thresholds for beekeepers to use when making management decisions for colony treatment. Colonies deemed below a treatment threshold may show mite populations significantly above the threshold two weeks later when sampled in late summer and early fall. The association between brood and mite distribution also suggests that brood frame manipulation might provide an effective management tool for altering mite distributions for targeted treatment approaches.
Figure. Varroa mite distribution in Hive 56 (A) in relation to capped and uncapped worker brood. (B) 2-D surface map and legend of mite numbers per cell and (C) 3-D map of mite density with a 2-D map overlay of the occurrences of brood (capped worker cells – red, uncapped worker cells – green) with respect to mite density.

13. Frost, E.H., D. Shutler & K. Hillier – EFFECTS OF A MITICIDE ON HONEYBEE MEMORY: IS THE CURE WORSE THAN THE DISEASE? WATCH VIDEO – Significant mortality from Varroa destructor has occurred in wild and managed honeybee populations. Although mortality is the clearest indicator of negative consequences, Varroa may have other subtle effects. For example, chemical treatments used to eliminate Varroa may interfere with the honey bees’ ability to properly integrate stimuli that elicit feeding, mating, colony defense, and communication behaviors.
We assessed learning and memory of honey bees exposed to tau-fluvalinate, the active ingredient in Apistan®, using a standardized Pavlovian insect-learning paradigm (proboscis extension reflex [PER]), that mimics learning in the natural environment. Honey bees are presented with a neutral stimulus, usually an odor, followed by a positive reward such as sugar water. Honey bees learn to extend their proboscis when exposed to the odor, in the absence of a reward, because the odor predicts the presence of food. Stressors, such as pesticides may reduce the frequency of PER, suggesting impaired learning (e.g., Abramson et al., 2004 Environ. Entomol. 33:378-388; Decourtye et al., 2005 Arch. Environ. Contam. Toxicol. 48:242-250).
Forager honey bees were collected in Nova Scotia, Canada in August/September 2009 and immobilized with only their antennae and mouthparts free. Tau-fluvalinate, dissolved in 1.25 µL of acetone, was applied dermally (thorax) or orally (proboscis) at concentrations of 0.125 µg (estimated to be daily exposure per bee in treated hives [Johnson et al., 2009 J. Econ. Entomol. 102:474-479]) or 1.25 µg. Controls were treated with 1.25 µL of acetone. Bees were trained to perform PER (training trials), and then tested for retention of odor memory 24 hours later (extinction trials).
Lower dose treatments had no significant effect on mortality or PER during training or extinction. At the 1.25 µg dermal dose, mortality was significantly higher in treated honey bees than controls at both 3 and 24 hours post treatment (p = 0.001 and p < 0.0001, respectively). Controls had a significantly higher average number of PER responses to odor cues during training (p = 0.05); there was no significant effect during extinction trials (p = 0.08).
We are also quantifying how tau-fluvalinate is partitioned within the honey bee body, and the relative concentrations. Chemical residues are evaluated using gas chromatography mass spectrophotometry by isolating the head and thorax and placing them in hexane to extract tau-fluvalinate. Quantities of tau-fluvalinate are measured by the size of the peaks on the chromatography output relative to a standard curve. Preliminary results suggest tau-fluvalinate enters the honey bee circulatory system after dermal contact. Honey bees with a dermal application (thorax) of tau-fluvalinate also have traces of the chemical in their head. Detoxification may also occur over time, with decreasing levels of tau-fluvalinate present in honey bee tissues over a 24 hour period.
Ultimately, this research will lead to standardized methods to evaluate suitability of mite treatment programs and potential sublethal effects of chemicals on honeybees. Bees worldwide are exposed to both mite and acaricide stressors, so results of this research will be applicable globally.
14. Guarna, M.M., A. Methalopoulus, S. Pernal & L.J. Foster- ANTENNAE PROTEINS AS MARKERS OF DISEASE RESISTANCE – A main goal of our APIS project (Apis mellifera Proteomics of Innate resistance) is to develop tools to facilitate selective breeding of stocks resistant to disease. These tools will be based on the discovery of proteins with levels that correlate with the ability of honey bees to resist or tolerate disease. In a collaborative effort of the proteomics team at UBC in Vancouver, BC and the AAFC Research Station in Beaverlodge, AB, we have analysed protein expression in honey bees with different level of resistant to American foulbrood (AFB). In particular, we investigated whether the relative quantity of proteins isolated from honey bee antennae was associated with field traits. Preliminary analysis of the data showed that the quantity of selected proteins was indeed related with at least one of the field traits, hygienic behavior. These proteins are our first potential markers of disease resistance and we are now performing follow up experiments to confirm these markers and investigate their heritability.
15. Hood, W.M. & B. Tate –FREEMAN SMALL HIVE BEETLE TRAP INVESTIGATIONS – The Freeman Beetle Trap was field tested at Clemson University, South Carolina in 2009. The trap consists of a hive bottom made of wood and screen that allows beetles to freely enter a removable plastic tray (partially filled with vegetable oil) below.
The primary objective of this research project was to compare the number of adult beetles killed in the Freeman Trap versus the Hood Trap (Nolan & Hood, 2008 J. Apic. Res. 47(3):229-233) during one full season. The Hood trap was secured into a shallow frame with trap-top flush with frame top bar and placed in the top honey super to allow convenient beekeeper access. The other objective was to measure and compare other colony parameters including adult bees, capped brood, honey, and varroa mites during the season.
Four apiaries were setup in the CU Experimental Forest. Six test colonies were established in each apiary with two-pounds package bees (Wilbanks Apiaries Inc., Claxton, Georgia) each on 6 April. On 12 May, colonies were randomly selected to receive one of three treatments: Freeman Trap, Hood Trap, or no trap (control). Treatments were replicated twice in each apiary. All 24 test colonies were fitted with Freeman Trap hive bottoms.
Freeman and Hood traps were serviced at 2-week intervals through 28 October by removing and counting dead beetles and replenishing traps with vegetable oil (Freeman Traps) or cider vinegar/mineral oil (Hood Traps). Each test colony received a 3-day survey for beetles and mites at 6-weeks intervals through 9 November by placement of a clean Freeman Trap tray with fresh vegetable oil and a varroa mite sticky board. Colony parameters including bees, capped brood, and honey were measured at 8-week intervals through 8 November. An end of season total “colony shakeout” of beetles on a white plastic table was conducted on all colonies on 16 November to count adult beetles remaining in colonies.
Four summations of beetles killed in the Freeman and Hood Traps (2 Jul, 14 Aug, 22 Sep, and 28 Oct) were compared. Significantly more (P<0.05) beetles were killed in the Freeman Trap on the first and second summation dates, and significantly more (P<0.05) were killed in the Hood trap on the final summation date. The Freeman Trap survey 2 on 20 Aug yielded a significant (P<0.05) increase in beetles killed in the Hood trapped colonies and the control colonies compared to the Freeman Trap colonies. There was a significant (P<0.05) increase in beetles killed in the control colonies versus the Freeman Trap colonies on the 3rd survey date, 28 Sep. There was no significant difference (P=0.06) in the mean number of beetles counted during the total colony shakeout of beetles (Freeman/50, Hood/71, and control/80). There were no overall treatment differences in colony parameters: adult bees, capped brood, honey, or varroa mites.
These preliminary investigations suggest that the Freeman Trap proved to be a more efficient trap based upon the number of beetles removed from the colonies. The low mean number of beetles (80) remaining in the control colonies at the end these investigations suggests that traps may have provided “trapping sinks” in test apiaries, thereby reducing the overall beetle numbers in all test apiaries, including control colonies.
16. Huang, Z.Y., K. Ahn, J. Riddle & J. Pettis – EFFECT OF TRANSPORTATION ON HONEY BEE PHYSIOLOGY – WATCH VIDEO – Despite the requirement of long distance transportation of colonies for almond pollination, we understand little of the effects of the long distance transportation on bees. We conducted three trials to study the effects of transportation on honey bee physiology. For each trial, newly emerged bees from one colony were split into two groups and introduced into a M (migratory) or S (stationary) group – these M and S colonies form a colony pair (CP) with similar genetics but different experience in transportation. One trial was conducted in CA/FL, whereby S bees stayed in Bakersfield, CA but M bees were moved to FL (4,000 km, n=12 colonies / group). One trial was done in Boston, GA, where the M group were transported to MI, then back to GA (3,250 km, n=12 colonies / group). In both trials, bees were about one week old and experienced transportation 3-5 days old. A third trial was in E. Lansing, MI, where the M group was transported for 900 km per day for 3 days (n= 6 colonies / group). In the first 2 trials, only one cohort of bees were age marked before transportation, but in the 3rd trial, bees were sampled at 7 and 17 day olds (they experienced transportation when they were 3-6 and 13-16 days old, respectively).
We measured the following parameters: juvenile hormone titers (JH) in hemolymph, lipid content in the abdomen, total protein in head or thorax, and sizes of hypopharyngeal gland (HPG) acini. HPG sizes were significantly smaller in migratory colonies. These were true for all three locations, and also for young and old bees in the Michigan trial (Table). This might be due to workers unable to consume pollen normally while being transported, which affected their gland sizes adversely. All other parameters (JH, lipids and total protein in heads or thorax) were either not as sensitive, or inconsistent. We were surprised to find that 1) JH titer was not significantly higher in the M group, and 2) that 17 day-old bees were still affected by migration, even though their HPG should have attained maximum sizes around day 8-12.
Table – Brief summary of analysis of variance (ANOVA) and analyses by individual colony pairs (CP). NS=no significant, with P>0.05.
| CA | |||
| JH | lipids | Head/thorax protein | HPG sizes |
| 2 out of 11 CP significant. ANOVA: NS | 2 out of 4 CP with higher lipids in S, the other 2 reversed. ANOVA: NS | Not measured | 7 out of 9 CP showed larger glands in S group. ANOVA: P<0.01 |
| GA | |||
| 2 out of 12 CP significant. ANOVA: NS | 1 out of 4 CP with higher lipids in S. ANOVA: NS | 9 CP analyzed. ANOVA: NS for thorax protein, but P<0.01 for head protein. | 4 out of 4 CP showed larger glands in S group. ANOVA: P<0.01 |
| MI (Young cohort, 7 days old) | |||
| 2 out of 6 CP had higher JH in M. ANOVA: NS | 5 out of 6 CP with higher lipids in S. ANOVA: P<0.01 | 6 CP analyzed. ANOVA: NS | 4 out of 6 CP showed larger glands in S group. ANOVA: P<0.01 |
| MI (older cohort, 17 days old) | |||
| 2 out of 6 CP had higher JH in M. ANOVA: NS | ANOVA: NS | 6 CP analyzed. O ANOVA: NS | 3 out of 6 CP showed larger glands in S group. ANOVA: P<0.01 |
17. Johnsonc, R., L. Petersc, B. Siegfriedd & M.D. Ellisc – DRUG INTERACTIONS BETWEEN IN-HIVE MITICIDES AND FUNGICIDES IN HONEY BEES Watch Video – Beekeepers must often resort to using varroacides to reduce parasitic varroa mite populations in honey bee colonies. The utility of varroacides depends on honey bee tolerance of these pesticides at dosages that kill varroa mites. Honey bee tolerance of three commonly used varroacides – coumaphos (Checkmite+TM), fenpyroximate (Hivastan TM) and tau-fluvalinate (Apistan TM) – appears to be due to bees’ capacity to detoxify these pesticides through cytochrome P450 monooxygenase activity.
However, a bee’s capacity for detoxification appears to be compromised when exposed to multiple varroacides simultaneously – a likely situation given the high levels of varroacide contamination reported in beeswax. Bees treated with a sublethal dose of coumaphos were 14 times more susceptible to tau-fluvalinate, as measured by the change in LD50. Similarly bees exposed to sublethal doses of fenpyroximate were 7.6 and 5.6 times more susceptible to coumaphos and tau-fluvalinate, respectively. Bees are also frequently exposed to fungicides applied to crops since fungicides are commonly considered safe for bees and there are few restrictions on their application during bloom. However, some fungicides may affect a bee’s ability to tolerate other pesticides, including varroacides. Honey bees pre-treated with pyraclostrobin and boscalid (both components of Pristine TM) or chlorothalonil (Bravo TM) were not more susceptible to any varroacides tested. Pre-treatment of bees with the fungicide prochloraz, however, increased the toxicity of coumaphos (72 times), fenpyroximate (23 times) and tau-fluvalinate (1118 times). These results confirm earlier work demonstrating that ergosterol biosynthesis inhibiting fungicides, a class of fungicides that includes prochloraz, can inhibit cytochrome P450-mediated detoxification of pesticides in honey bees. Based on these laboratory findings, it may be prudent for beekeepers to avoid repeated use of P450-interacting varroacides – tau-fluvalinate, coumaphos and fenpyroximate – and to avoid using these varroacides when bees are likely to come into contact with P450-inhibiting ergosterol biosynthesis inhibiting fungicides.
18. Kather, R. & S.J. Martin – FRIEND OR FOE? NESTMATE RECOGNITION IN THE HONEY BEE APIS MELLIFERA – WATCH VIDEO – Honey bees can tell nestmates from non-nestmates and chase away any intruder trying to enter their hive. Despite this, one of the honey bee’s major and most lethal parasites, the Varroa mite, frequently enters and travels between hives. To determine how Varroa is able to overcome the bees’ detection system we need to better understand how bees recognize each other. Insects use their sense of smell to identify other insects. Every insect produces a set of chemicals on their ‘skin’, so-called cuticular chemicals, and usually insect species differ in the type of chemicals they produce (Howard & Blomquist, 1982 Ann. Rev. Entomol. 27:149-172). In the social insects such as wasps, hornets and bees, colonies of the same species produce the same types of chemicals, but chemical quantities vary between colonies forming a colony-specific odor. This way, one insect can identify another insect, i.e. which species or colony it belongs to, by ‘reading’ the chemicals on that insect’s skin. Chemically, Varroa ‘looks’ like a honey bee, which partly explains why it remains undetected in the hive, but how does it overcome the bees’ colony recognition when it moves between hives? To answer this question we need to identify the compounds bees use to identify nestmates.
Previous studies have suggested two candidate classes: the fatty acids and the alkenes (Breed & Stiller, 1992 Anim. Behav. 43:875-883, Dani et al., 2005 Chem. Senses 30:477-489). But chemical evidence to support this is still missing. Our research was the first to see whether nestmates had similar quantities of fatty acids/alkenes and whether colonies varied in these quantities, which we would expect from colony recognition compounds. Our data suggests that this is indeed the case for the alkenes, but not for the fatty acids. Fatty acid quantities varied considerably between nestmates and thus cannot function in nestmate recognition. Every colony had its own alkene profile (Figure). The next step of our research will be to investigate whether and how Varroa mimics these recognition compounds to stay undetected in the colony. Only by better understanding the Varroa-honey bee system can we begin to disrupt this system of manipulation and design new and more effective treatments to combat this enemy common to bee and man.
19. Melathopoulos, A.P., S.F. Pernal, A. van Hagaf & L.J. Foster VARIABILITY AND CORRELATIONS AMONG FIVE TRAITS ASSOCIATED WITH AMERICAN FOULBROOD (AFB) RESISTANCE IN A CANADIAN BREEDING POPULATION WATCH VIDEO – The demonstration of AFB resistance in the 1930s lead to the discovery of several resistance traits (Spivak & Gilliam, 1998 Bee World 79:124-134, 169-186). The heritability of these traits in commercial breeding populations, their correlation and their relative contribution to overall resistance, however, remains poorly understood. For this reason we compared the distribution of AFB traits within a breeding population.
We assembled colonies in a common apiary headed by queens from eight different regions (New Zealand, Chile, Hawaii, California, British Columbia, Alberta, Saskatchewan, and Ontario). These were tested for 1) Hyg Beh: hygienic behavior, 2) Larval AFB: the percentage of in vitro reared larva with AFB after being fed Paenibacillus larva spores, 3) Nurse Spore: the retention of spores by nurse bees fed spore-containing syrup, 4) Patch AFB: the percentage of first-instar larvae in comb developing AFB in situ after inoculation and 5) Comb AFB: the number of AFB cells in colonies following inoculation with AFB-infected comb.
There were five significant correlations among the traits (Figure), the strongest being between four related traits; Hyg Beh measured at 24h versus 48h, and Nurse Spore from whole colony tests in 2008 versus cage tests in 2009. More notable, however, were correlations among a number of seemingly unrelated traits. Principal component analysis revealed that among these later traits Hyg Beh and Patch AFB (14d after infection) loaded diagonally on the first component, while Larval AFB loaded diagonally to Patch AFB (7d after infection) on the second component. This suggests that Hyg Beh and Larval AFB may work synergistically but at different stages of disease’s development. Nurse Spore loaded strongly on the third component suggesting the trait is unrelated to the other traits.
Our next step will be to estimate quantitative genetic parameters for each trait by assessing them among an F1 generation produced through a partial diallele cross of selected colonies.
20. Nasr, M.E. & A.J. Williamson – VARROA HAND SHAKER: A SIMPLE FIELD METHOD FOR MONITORING VARROA MITE INFESTATIONS – Determining varroa mite’s infestation levels is critical when making decisions to implement integrated pest management practices for varroa control. Current methods are time consuming and expensive. A simple fast reliable method to monitor the population of varroa mites in honey bee samples in the field was developed. Two 500 ml transparent plastic spice jars were modified to make the “Varroa Hand Shaker”. The centers of two closure lids of the plastic jars were removed to accommodate a 7 cm diameter circle made of 8 mesh-screen. The screen was placed in between the two closure lids, and then lids were glued back together to form a strainer-closure that can keep the two jars connected mouth to mouth.
A sample of 300-400 bees from brood combs was collected in one of the varroa hand shaker jars that contained 250 ml of 70% alcohol. This sample of bees filled about 2.25 cm in the jar. Then, the second jar with the strainer-closure was screwed back on the jar containing bees. The varroa hand shaker was vigorously shaken up and down for 40-60 sec. To determine varroa infestation levels, the varroa hand shaker was flipped upside down to keep the bees on the top of the screen and allow the mites and alcohol to pass through the screen into the bottom jar. The number of mites collected in the bottom jar was counted. The same sample was shaken using an orbital lab shaker at 175 rpm for 15 min to compare results. Bees and mites were washed using a strainer and collected mites were counted. The percentage of infestation based on the number of bees/ sample was calculated for each of the tested methods. The percentages of infestation were determined in 1,590 bee samples. The two methods were significantly correlated (r=, P<0.0001) (Figure). A correction factor as indicated in the Figure can be used to correct the percent infestation relative to the standard orbital shaker method. The developed varroa shaker method is currently widely used in Alberta, Canada by commercial beekeepers to determine the infestation of varroa mites because it is simple, accurate, and inexpensive. It also requires very little time.
Financial support by Alberta Beekeepers, Agriculture Crop Industry Development Fund Ltd. (ACIDF), Poelman apiaries, Bayer Cropsciences Canada , Pioneer Hi-Bred, Hy-tech, Monsanto, Sothern Alberta Beekeepers Association, and Agriculture and Agri-Canada; Growing Forward.
21. Nasr, M.E., S. Muirhead, R. Panasiuk & L. Vandervalk – HONEY BEE WINTER KILL FROM 2007-2009 IN ALBERTA, CANADA: ARE OUR BEES HEALTHY? – In Alberta, Canada, beekeepers reported higher than average winterkill in overwintered bee colonies in spring 2007-2009. To determine the extent of winterkill, Alberta Agriculture and Rural Development conducted an annual survey of 100-112 commercial beekeepers with 400 or more colonies in the past three years. The percentages of beekeepers who responded to the surveys were over 75%. Survey results show that the beekeepers reported 30% loss per year in 2007, 2008, and 2009. The surveys also revealed that 15% the surviving colonies were weak with less than 3 frames covered with bees. The average percentage winterkill plus weak colonies was 45%. The reported winterkill and weak colonies were the lowest in southern Alberta region and the highest in the Peace River region.
The beekeepers’ responses in these surveys indicated that over-winter losses in Alberta during the past three years may be attributed to a combination of several potential causes: 1) Varroa mites unexpectedly developed resistance to applied miticides; consequently, mite populations were higher than normal in bee colonies and damaged winter bees, 2) beekeepers reported higher than normal incidence of Nosema-like symptoms which was shown to be positively correlated with winterkill and winterkill plus weak colonies, and 3) unusual weather conditions in fall and winter reduced the production of winter bees and increased the stress through winter months on wintering bees. Disappearance or starvation of honey bees were not major factors in reported high overwinter losses in Alberta. The data also showed that colony losses cannot be attributed the Colony Collapse Disorder currently described in the United States.
Honey bee colonies (12-60/beekeeping operation) were examined in fall of 2007, 2008 after treatment of colonies for varroa and Nosema. The percentage of varroa infestation was determined in 300-400 bee samples/colony that were collected from the brood area. The abundance of Nosema spores in a sample (30 bees/colony) collected from honey combs was calculated. Based on these two criteria in 2007, it was estimated that 10% of the beekeeping operations were healthy. These operations had varroa and Nosema below the economic threshold (1% varroa infestation and 1 million Nosema spores/bee). The rest of the colonies had varroa, Nosema, tracheal mites or varroa and Nosema above the thresholds. Similar results were found in 2008.
In fall 2009 results of examining 24-60 colonies/commercial beekeeping operation showed that 79% of beekeeping operations had varroa and Nosema below the economic thresholds. The reported improved bee conditions were due to successful use of Apivar, a newly registered miticide for varroa control as well as using fumagillin for Nosema treatment. Results showed that the percentages of varroa infestation and abundance of Nosema were significantly correlated to colony mortality. These results confirm previously reported possible causes of high winterkill in beekeepers’ surveys. Attention to disease prevention and surveillance for early detection were critical points for colony survival and improving bee health.
Research sponsored by Alberta Beekeepers, Agriculture Crop Industry Development Fund Ltd. (ACIDF), Poelman apiaries, Bayer Cropsciences Canada , Pioneer Hi-Bred, Hy-tech, Monsanto, Sothern Alberta Beekeepers Association, and Agriculture and Agri-Canada; Growing Forward.
22. Ostiguy, N. – A PEEK AT THE DISTRIBUTION OF VIRUSES IN STATIONARY HONEY BEE COLONIES IN THE U.S. – A seven state (CA, FL, ME, MN, PA, TX and WA) long-term study of 30 stationary colonies per location began in spring 2009 to track colony health and longevity using queens from the same source to control for genetic variation. This study will provide epidemiological data to characterize the similarities and differences in colony health across the U.S. through time. This is a report on the preliminary virus results for six of the seven locations (no data on CA available).
It was not practical to establish colonies at the same time of year due to differences in climate. Package installation matched forage availability – February/March in the southern locations and April/May in the northern locations. Packages were not obtained from the same source as virus incidence and prevalence from a single package supplier varies among packages obtained at the same time (unpublished, PSU). Two pairs of apiaries, CA–MN and ME–PA, obtained bee packages from the same supplier.
The viruses obtained from the bees initial package samples varied significantly by location (Table). A significantly higher prevalence of Black queen cell virus was observed in the Minnesota packages (p=0.00000001), while DWV and SBV were significantly higher in Texas (p=0.00000001) and Washington (p=0.00000001), respectively. The prevalence of Israeli acute paralysis virus was low but prevalence in Washington was the highest (p=0.00000001). The bees in the packages received by Pennsylvania, even though the supplier was the same as those bees received by Maine, had a higher prevalence of BQCV and DWV but SBV prevalence was lower; the differences were 38%, 19% and 14% respectively.
Seasonal changes occurred in virus prevalence. Deformed wing virus prevalence increased from 55% to 96% of bees tested from Maine. The bees in Maine began with the lowest prevalence of DWV (55%) but by August the prevalence of DWV was the highest (96%). In our preliminary data, Maine also was experiencing the highest level of Israeli acute paralysis virus (12%). This preliminary data indicates that virus prevalence varies by region and in time. We also have evidence that the viruses found in packages from the same source are not similar.
Table – Regional Prevalence of Honey Bee Viruses* Black queen cell Deformed Wing Israeli acute paralysis Sacbrood
|
Table – Regional Prevalence of Honey Bee Viruses* |
||||
|
|
Black queen cell |
Deformed Wing |
Israeli acute paralysis |
Sacbrood |
|
Florida |
20%** |
83% |
0% |
20% |
|
Maine |
29% |
55% |
0% |
24% |
|
Minnesota |
87% |
56% |
2% |
16% |
|
Pennsylvania |
67% |
74% |
1% |
10% |
|
Texas |
24% |
96% |
0% |
0% |
|
Washington |
58% |
61% |
7% |
77% |
*n = 521 ** Percent of positive samples
23. Parker R., M.M. Guarna, S. Pernal & L.J. Foster- APIS MELLIFERA PROTEOMICS OF INNATE RESISTANCE (APIS) WATCH VIDEO – The APIS project focuses on two diseases affecting honey bees in North America, American foulbrood (AFB) and Varroa mites. Although treatments are available, increased resistance and the concern of chemical residues makes selective breeding of resistant bee stock the most desirable solution. Our goal is to develop tools for accelerating and strengthening the selection process. These tools will be based on protein markers that indicate resistance and can predict whether a honey bee stock will be more likely to resist or tolerate disease. In our first year, we sampled diverse stocks (Figure), tested behavioural and physiological field traits, and are using Mass Spectrometry-based proteomics to compare protein expression. Analysis of ca. 500 quantified proteins in each tissue showed significant protein differences that were related to the characteristics of the bees sampled. For the 2009-2010 experiments, we acquired additional queens and have implemented a mating design to study heritability of the field traits and the protein markers associated with them. We are also collaborating with Jeff Pettis, USDA, to investigate the economic impact of disease on the beekeeping industry. Our recent progress will be presented.
24. Pernal, S.F., A. Ibrahim & A.P. Melathopoulos – DISINFECTION OF NOSEMA CERANAE-CONTAMINATED COMB BY IRRADIATION, ACETIC ACID FUMIGATION AND HEAT – Watch Video Nosema ceranae is an endoparasite first described from Apis cerana (Fries et al., 2006 Eur. J. Protistol. 32:356-365) that has subsequently spread to populations of Apis mellifera in Europe and throughout the world (Higes et al., 2006 J. Invertebr. Pathol. 92:93-95; Klee et al., 2007 J. Invertebr. Pathol. 96:1-10). In North America, the parasite has been associated with Colony Collapse Disorder in the U.S. (van Engelsdorp et al., 2009 PLoS ONE 4(8):e6481) and implicated in heavy wintering losses in Canada (Currie et al., 2010 J. Apic. Res. 49:104-106).
Though mechanisms of transmission for N. ceranae are not well understood, we hypothesized that decontamination of comb could be an important non-chemotherapeutic technique for managing this organism. We undertook an examination of new techniques of comb sterilization in addition to those previously demonstrated to be effective at suppressing Nosema apis (Bailey, l957 Am. Bee J. 97:24-26; Cantwell & Shimanuki, 1969 Am. Bee. J. 109:52-54).
Frames containing honey comb were sprayed with an aqueous suspension of N. ceranae, so that each colony received a dose of 4.51 × 108 spores. Inoculated brood chambers were allocated to four groups, each with 12 replicates, receiving one the following treatments: 1. Irradiation – 10 kGy of electron beam radiation. 2. Acetic Acid Fumigation – 480 mL of 80% (v/v) acetic acid. 3. Heat – 24 h at 49 ± 0.1°C. 4. Inoculated – no disinfection. A fifth, non-inoculated, treatment consisted of brood chambers receiving neither inoculation nor disinfection. Sixty 1-kg New Zealand packages were hived in the brood chambers on 2 May 2009. Colonies were sampled for N. ceranae spores by collecting a minimum of 30 adult bees on a weekly basis from 2 May to 4 June 09, after which time sampling occurred biweekly, then monthly during winter. Colony growth was evaluated by measuring adult worker population and sealed brood on 30 July and 30 August; honey production was weighed.
Thirteen days after hiving packages, spore levels within inoculated, untreated colonies rapidly proliferated to 2.4 ± 0.4 x 106 spores per bee while spore levels in irradiation, acetic acid fumigation and heat treatments remained below 167,000 spores. Nonetheless, by 21 May the acetic acid fumigation and heat treatments appeared less effective at suppressing spores than the irradiation treatment which remained similar to non-inoculated colonies. Separation among treatments diminished until 16 July when spore levels among all treatments, including those untreated, remained below 100,000 spores per bee. Considerable variability and increases in spore levels for the acetic acid fumigation and heat treatments were observed during the fall and early winter months.
Significantly more adult bees (F=4.52; df=4,52; P=0.0033) were found on the 3 July assessment in the irradiation versus heat treatment, with the fumigation, non-inoculated and inoculated treatments being intermediate in number. No significant differences among treatments were found for the area of sealed brood on this date, or for both parameters on 30 August. Honey production did not vary significantly among treatments.
In general, the acetic acid fumigation, heat and irradiation treatments all showed some suppression of the spring peak of N. ceranae spore development in bees, though suppression was more immediate and complete for irradiated comb. Only the irradiated treatment maintained spores at levels similar to non-inoculated colonies during the entire study.
25. Peters, L.A., R.M. Johnson, M.D. Ellis & B. Siegfried – EFFECTS OF IN-HIVE MITICIDES ON HONEY BEE DRONE SURVIVAL AND SPERM VIABILITY – Miticidal drugs are commonly used by beekeepers to control infestations of the devastating honey bee (Apis mellifera) pest Varroa destructor. One of these compounds, coumaphos, has been documented to affect the sperm viability of drone honey bees in a test based on prolonged exposure to Checkmite+ strips. In our study, newly emerged drones were treated topically with varying concentrations of six miticides: tau-fluvalinate, coumaphos, amitraz, fenpyroximate, oxalic acid and thymol. Drones were marked with paint and reintroduced to free-flying hives where they were allowed to become sexually mature. After two to three weeks drones were recaptured, semen was collected and sperm viability tested with a SYBR14 / propidium iodide fluorescent assay. No differences in sperm viability were noted in the semen of drones receiving any of the miticide treatments; however, thymol treatments positively affected drone recapture, and fenpyroximate treatments negatively affected the likelihood of drone recapture. While our tests found no effect on sperm viability for a single dose exposure, 5 of the 6 treatments are applied by beekeepers in delivery systems that expose bees over an extended time period, and prolonged exposure to miticides may reduce sperm viability where single dose exposures did not. The effects of thymol and fenpyroximate on drone recovery (survival) have not been previously reported.
26. Rogers, R.E.L., G.R. Williams, C. Lam, D.L. Fischer, & D.E. Hackenberg – MOVENTO®, CITRUS AND HONEY BEES: REPORT ON A SUCCESSFUL COOPERATIVE STUDY – The Asian citrus psyllid (Diaphorina citri) is the single greatest insect pest to the global citrus industry. Infestation typically results in withdrawal of large quantities of foliage sap and transmission of bacteria responsible for greening disease. If untreated, infestation will result in premature defoliation, poor fruit production, and eventual death of trees. Citrus growers have numerous options for controlling the Asian citrus psyllid, however, many are not bee-safe and must be used before or after bloom, which is not optimal for psyllid control. A new promising candidate for the job is Movento®, a.i. spirotetramat. Movento® is a unique two-way systemic foliar insecticide that is effective on psyllid, and is not believed to produce harmful effects to non-target species such as honey bees. In collaboration with Bayer CropScience, the National Honey Bee Advisory Board, the US EPA, Florida Department of Agriculture and Consumer Services and the USDA, we investigated the potential effects of Movento® on western honey bee (Apis mellifera) colonies when applied to citrus during bloom using our jointly developed protocol for western honey bee colony effects studies. Twelve colonies were placed at the edge of each of two citrus groves at the start of bloom and removed after bloom finished; one grove received a single application of Movento® at full label rate of 10 oz/ac (730 ml/ha) and the other received no treatments. Colonies were assessed for strength and health (i.e., adult and brood populations, food stores, pests, and diseases), and pollen, honey and wax samples were collected and analyzed for presence of residues of spirotetramat and other pesticides. After the conclusion of field work in Florida, the colonies were returned to commercial beekeeping operation and transported to northern states for use in pollination of several crops. Follow-up assessments of the health of these colonies were performed in May in Pennsylvania after apple pollination, in June in Maine after blueberry pollination, and in October in Pennsylvania after late summer pumpkin pollination. There were no significant differences between the control and treatment groups of hives during the in-citrus phase of the study. Both groups of hives started experiencing high losses of colonies between the blueberry and fall colony assessments. Varroa mite and Nosema counts were high in both groups of hives throughout most of the study period, and deformed-wing condition accounted for almost one-third of intra-hive mortality before leaving citrus. Residues of spirotetramat in bee-relevant matrices were detected in small amounts for only a few days post-application and were not-detectable in later samples. It appears there is a high degree of safety with Movento® use during citrus bloom. It appears likely that the primary causes of the high colony losses by fall, in both groups of hives, were Varroa destructor, Nosema spp., deformed-wing virus, queen issues, and multiple and various other pathogens to a lesser degree.
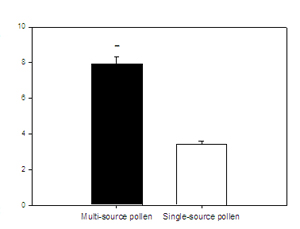
27. Sagili, R.R. & C. Breece – EFFECTS OF POLLEN QUALITY ON HONEY BEE NUTRITIONAL STATUS AND COLONY GROWTH – Nutritional stress is one among several potential factors attributed for colony collapse disorder. In the wake of deteriorating honey bee health, honey bee nutrition has attained greater importance. Pollen is the sole source of protein for honey bees and is vital for their development and survival. Large monoculture and specialized greenhouse farming systems result in restricted choice of pollen diet in honey bees (Schmidt et al., 1995 J. Econ. Entomol. 88:1591-1595). Each year large numbers of colonies from all around the country are shipped to California for almond pollination, where bees predominantly rely on almond pollen to fulfill their protein requirement. Little is known about effects of single source pollen consumption for extended periods on honey bees. Here we examined and compared the effects of single-source pollen consumption versus multi-source pollen on honey bee nutritional status and colony growth.
Six frame nucleus colonies were used for this experiment. A pair of colonies was derived by dividing a single colony and were headed by a naturally mated sister queens. A large flight cage partitioned in segments was used for this experiment. Colonies were equalized before start of the experiment and all existing pollen was removed. There were two treatments: 1) single-source pollen and 2) multi-source pollen. Powdered pollen was packed into the comb cells and surface sprayed with 50 % sugar syrup (Dreller & Tarpy, 2000 Anim. Behav. 59:91-96). Each week 100 newly emerged paint marked bees were introduced into each experimental colony. Each week 20 nurse bees were obtained from each colony for hypopharyngeal gland protein estimation and comb area occupied by eggs, larvae, pupae, pollen, honey and empty space were measured. Lipid content and emergence weights of newly emerged bees were also measured. Preliminary results indicate that nurse bee hypopharyngeal gland protein content and colony growth in single source pollen treatments were significantly low compared to multi-source pollen treatments (P < 0.01 and P < 0.05 respectively) (Figure).
28. Sheppard, W.S., B.A. Kahkonen, & K.E. Northfield – SEASONAL ABUNDANCE OF NOSEMA AND TRACHEAL MITES IN CAP STATIONARY APIARIES – As part of a larger Coordinated Agricultural Project on honey bee colony health, we established 7 stationary apiaries across the United States. The locations were chosen to represent a diverse set of ecological conditions across the country and sites were selected in California, Washington, Maine, Minnesota, Florida, Texas and Pennsylvania. The apiaries of 30 colonies each were established in Spring 2009 with new woodenware, plastic foundation and package bees. As soon as practicable, sister queens of an Italian strain from a California queen producer were obtained and all colonies were requeened, to provide a common genetic foundation for all apiaries. Colonies were maintained using standard beekeeping practices appropriate for each location, but without chemical or antibiotic treatments.
I report here the preliminary results for Nosema and tracheal mite levels found in the stationary apiaries in 2009 based on monthly sampling. The samples were collected in ethanol and shipped to the Washington State Honey Bee Diagnostic Laboratory. Tracheal mites were determined through dissection and microscopic examination of tracheae from 20-30 bees from each colony and reported as percent infestation (# infested bees / # bees in sample). Nosema spore counts were determined by homogenization of abdomens from 50 workers and a standard protocol that involved serial dilution and microscopic examination of diluted material using a hemocytometer. Spore levels are reported as mean # spores per bee. Nosema species determinations were made on a subset of samples from each location using a standard PCR-based protocol, restriction enzyme digestion and gel electrophoresis.
Tracheal mite levels varied across locations and month sampled, and preliminary data are reported from samples taken from April to August (Figure). Tracheal mite levels in the three northern locations were highest in July, with the highest mean % infestation of bees reaching around 26% in Maine.
Molecular analysis of representative Nosema samples revealed that only Nosema ceranae occurred. Nosema spore levels also varied by location and month, but were generally highest in May and quite low in July and August (Figure). Overall, the highest mean spores/bee levels were found in the Minnesota (3.4 million spores/bee) and Washington (1.4 million spores/bee) stationary apiaries sampled in May.
The samples taken early in 2009 likely reflected the starting infestation levels of the package bees, while subsequent samples reflect infestation levels that became established in the replacement bee populations produced by the genetically similar queens used at all sites. Additional data from samples collected in Fall 2009 and Spring 2010 will provide a more accurate picture of the seasonality of Nosema and tracheal mite levels occurring at these stationary sites. Sampling and monthly pathogen/parasite evaluations of surviving colonies will continue through 2010, to better assess seasonal variation and potential geographic differences among stationary apiary locations.
29. Smart, M.D. & W.S. Sheppard – DISTRIBUTIONS OF NOSEMA CERANAE AND PARASITE INTERACTIONS IN PACIFIC NORTHWEST HONEY BEES – Nosema ceranae is a microsporidian parasite that infects the midgut of honey bees. Its pathogenic effects on bees include decreased longevity, younger foraging age, greater hunger, and a suppressed immune system. Recent evidence suggests that this relatively newly recognized species is becoming more widespread throughout much of the world (Higes et al., 2006 J. Invertebr. Pathol. 92:93-95, Klee et al., 2007 J. Invertebr. Pathol. 96:1-10, Martin-Hernandez et al., 2007 Appl. and Env. Microbiol. 73(20):6331-6338, Williams et al., 2007 J. Invertebr. Pathol. 97(2):189-192).
N. ceranae may be more virulent than its better-known cousin, Nosema apis (Higes et al., 2007 J. Invertebr. Pathol. 94:211-217), and may have the ability to persist at higher levels throughout the year (Martin-Hernandez, 2007 Appl. and Env. Microbiol. 73(20):6331-6338). Another recent study, in which researchers fed honey bees both N. ceranae and N. apis spores, found that while exposure to N. apis induced a honey bee’s immune system to respond and fight the infection, N. ceranae had an immunosuppressive effect on honey bees (Antunez et al., 2009 Environ. Microbiol.doi:10.1111/j.1462-2920.2009.01953.x). This underscores the importance of studying the interactions between N. ceranae and other pests and pathogens that may come together to cause greater weakening and collapsing of honey bee colonies.
There were three main objectives of this research. First, we sought to examine the basic regional and seasonal distributions of Nosema apis and Nosema ceranae in the northwestern U.S. by surveying migratory and sedentary beekeeping operations. Second, to identify age trends in N. ceranae infection, we individually processed bees and quantified the N. ceranae infection levels of age cohorts of bees. Finally, we examined the interactions and deleterious effects between N. ceranae and the parasitic mite, Varroa destructor in the field. Migratory and stationary apiaries were sampled between February 2008 and September 2009 to quantify Nosema spp. infection levels. DNA was subsequently extracted from samples testing positive for high levels of Nosema spp. infection, and the species present was determined. Preliminary results indicate that Nosema ceranae is the predominant species present, being distributed widely in the PNW and detected in all months sampled. Nosema apis was also detected, but at a low rate.
Age cohorts (groups of bees emerging around the same time) were followed in five colonies to track Nosema ceranae infection as individual bee’s age. Forager bees (22-25 days old) had the greatest proportion of infected individuals and the highest levels of infection. This suggests that susceptible forager bees are most responsible for harboring the parasite; however it is still unclear how N. ceranae is transmitted in the hive environment.
Interactions between Nosema ceranae and the serious mite pest, Varroa destructor, were examined to better understand how these two important stressors might interact in the field. Additionally, a goal of this study was to determine if high N. ceranae infection levels could be maintained in colonies receiving weekly inoculations of N. ceranae spores.
Forty colonies, each undergoing one of four treatments, were sampled weekly for N. ceranae and Varroa destructor, and measures of strength and production were taken. As expected, colonies with low levels of N. ceranae and mites were significantly stronger at the end of the experiment than colonies with high levels of the two parasites. Colonies inoculated with N. ceranae weekly did contain bees with significantly higher infection levels. This method of artificial inoculating colonies with N. ceranae is potentially useful in future studies on colony level dynamics of ‘N. ceranae and other various pathogens and parasites.
30. Stoner, K.A. & B.D. Eitzer – PESTICIDE IN POLLEN TRAPPED FROM HONEY BEE HIVES IN CONNECTICUT – TWO YEARS OF RESULTS IN RELATION TO ACUTE TOXICITY DATA – In two years of measuring pesticides in trapped pollen from healthy hives in 3-4 locations around Connecticut, the authors found residues of 44 pesticides and pesticide metabolites. As a very rough first step in evaluating the significance of the concentrations of these pesticides in pollen, we gathered the available data on the contact and oral lethal doses that would kill 50 % of the tested population (LD50) of these pesticides from the U.S. Environmental Protection Agency and Agritox (France1) databases. Note that LD50 is typically measured with a single dose, evaluated over 24 -48 hours (U.S. EPA 19962). Not all of the pesticides found have acute toxicity data in these databases, including the most commonly detected pesticide, coumaphos.
In order to calculate a dose in micrograms per bee from the concentration data, we used the calculation of Rortais et al. (2005 Apidologie 36:71-83) that an adult worker bee would consume 65 mg of pollen, on average, during a period of 10 days as a nurse bee.
Using this calculation for the concentration of each pesticide found in a trapped pollen sample (mixed pollen collected over 3-4 days), we found only one sample in 2007 where the dose of pesticide was calculated to be over the oral LD50 – a sample with imidacloprid measured at 70 ppb, which calculates to 123% the oral LD50 from the Agritox database. Pesticides with residues over 10% of the contact or oral LD50 are given in the Table.
1Agence Française de Sécurité Sanitaire des Aliments (AFSSA). 2009. Agritox database. Base de données sur les substances actives phytopharmaceutiques. http://www.dive.afssa.fr/agritox/php/fiches.php
2 Ecological Effects Test Guidelines. OPPTS 850.3020. Honey Bee Acute Contact Toxicity. http://www.epa.gov/opp00001/chem_search/cleared_reviews/csr_PC-029088-20…
Table – Pesticide residues found in pollen that met or exceeded the contact or oral LD50.
|
Pesticide |
Maximum residue in a sample (ppb) |
Calculated dose to a nurse bee (µg/bee) |
% of contact LD 50 |
% of oral LD 50 |
|
Imidacloprid (2007) |
70 |
0.0046 |
10% |
123% |
|
Imidacloprid (2008) |
23 |
0.0015 |
3.4% |
40% |
|
Chlorpyrifos |
25 |
0.0016 |
16% |
1.5% |
|
Phosmet |
3720 |
0.242 |
23% |
No LD 50 |
31. Szalanski, A., J. Whitaker & P. Cappy – MOLECULAR DIAGNOSTICS OF NOSEMA CERANAE AND N. APIS FROM HONEY BEES IN NEW YORK – WATCH VIDEO – Molecular diagnostics of the invasive honey bee pathogens, Nosema ceranae and N. apis, were conducted on honey bees from New York. A total of 1200 honey bee samples were collected by NYSDAM bee inspectors, of which, 528 (44%), from 49 counties had a positive Nosema spore count. Spore counts were the highest in the Spring and decreased into the Fall. PCR genetic analysis on 371 spore-positive samples revealed that 96% were N. ceranae, 3% had both N. ceranae and N. apis, and 1% had N. apis. Nosema ceranae was more common in commercial operations relative to sideliner and hobbyist beekeepers. A preliminary molecular phylogenetic analysis of N. ceranae from New York revealed that it is identical to N. ceranae from Nebraska, South Dakota, Wisconsin, Europe and Australia.
32. Traver, B.E. & R. Fell – STATEWIDE SURVEY OF NOSEMA IN VIRGINIA HONEY BEE COLONIES – Virginia beekeepers have reported average colony losses of 30% over the past five years. The recent discovery of Nosema ceranae in the U.S. has raised concerns among beekeepers as to whether this new pathogen could be contributing to these losses. Since N. ceranae is thought to be more pathogenic and has been linked to colony collapse disorder in Spain (Higes et al., 2008 Environ. Micro 10:2659-2669; Higes et al., 2009 Environ. Micro Reports 1:110-113), we initiated a statewide survey of honey bee colonies in Virginia to determine the incidence of N. apis and N. ceranae. Samples were collected from 305 colonies around the state and included samples from Virginia Tech apiaries, as well as samples sent in by Virginia beekeepers and apiary inspectors. Samples were analyzed for Nosema using both spore counts and molecular techniques. Spore counts were made by crushing the abdomens of 10 bees in water and counting spores with a hemacytometer. Genomic DNA was extracted from worker abdomens (5) and analyzed by real-time PCR for the presence of Nosema. All samples were run in triplicate, using a multiplex real-time PCR assay developed in our laboratory that uses primers and probes specific to either N. apis or N. ceranae 16S rRNA small subunit. The assay was validated using known N. apis and N. ceranae DNA samples provided by the USDA. This PCR assay allows for both the identification of the Nosema species and an estimate of the level of infection.
The results of the spore count studies indicate that 37.5% of the hives in Virginia are infected with Nosema spores, and real-time PCR analysis of positive samples indicated that N. ceranae is the dominant species present. N. apis infections were observed at very low levels (3.6%) and occurred only as a co-infection with N. ceranae. Our data also indicate that traditional diagnoses based on spore counts alone do not provide an accurate indication of colony infections. We estimate that 55% of colonies determined negative by spore counts are infected with N. ceranae when analyzed by real-time PCR. The actual infection level of colonies in Virginia, based on molecular diagnosis for N. ceranae, was 72%.
Infection levels in hives were estimated using a cycle threshold value (CT). CT values are used as a relative indicator of the concentration of target DNA in a reaction. In hives that tested positive for N. ceranae, average CT values were used to diagnose a hive as having a low (CT above 32), moderate (CT 24-31), or heavy (CT less than 23) level of infection. Of the hives analyzed, 11% of hives were classified as a heavy infection, 16% as a moderate infection, and 73% as a low level of infection. Based on these results, we were able to provide beekeepers with advice on treating their hives, suggesting that beekeepers with colonies having a moderate to high level infection consider treating with Fumagillin B.
33. Traynor, K.S., R. Page & G. Amdam – EARLY ENVIRONMENT INFLUENCES THE BEHAVIORAL RESPONSE OF APIS MELLIFERA TO BROOD PHEROMONE – Brood pheromone (BP), a mixture of ten fatty acid esters given off by developing larvae, has previously been shown to both increase pollen foraging and prolong nursing behavior. Mutually exclusive effects of delayed foraging or precocious foraging have been ascribed to varying doses of BP. Why does the behavioral response of bees to the same pheromone differ at times?
New research indicates that prior experience can modulate the response to queen mandibular pheromone (QMP). Young bees are normally attracted to the queen and her pheromone, but if they have never previously experienced QMP, they are instead repelled by it just like foragers (Vergoz et al., 2009 PNAS 106:20930-20935).
Previous brood pheromone research has been conducted in small cages, nucleus colonies and full size hives. Our research using synthetic BP indicates that newly emerged (NE) bees placed directly into cages in an incubator respond to BP in the opposite direction than bees that have previously moved freely in a nucleus colony for two days (MA). This may help explain why previous BP results have often been contradictory.
Gustatory response scores (GRS) in the proboscis extension reflex (PER) test provide a window into future foraging choices. Pollen foragers typically have high scores, while nectar foragers typically have low scores. In response to both a high and low BP treatment, NE bees significantly increased their gustatory response scores (GRS) (Kruskal-Wallis: χ2 = 6.576, n = 2, P = 0.037). BP treatment did not significantly affect the GRS scores of MA bees, although the trend was in the opposite direction from the NE bees. Thus overall, there was a significant effect of bees (NE vs. MA) (Kruskal-Wallis: χ2 = 6.445, n = 1, P = 0.011). MA bees consumed significantly more of the 30% sucrose solution compared to NE bees (ANOVA: Bees F(1,76)= 65.618, P<0.001) and significantly less pollen (ANOVA: Bees F(1,72)= 5.716, P=0.019). There were no significant differences in mortality or water consumption.
Our results suggest the initial exposure to the hive environment primes bees to perceive BP differently than bees that have never previously encountered BP. The MA bees that have the opportunity of experiencing a hive environment must gorge on protein during those first two days of life. They have significantly heavier rectums than the NE bees, although they consume less pollen in the cages (Rectum weights ANOVA: Bees: F1,116 = 4.278, P= 0.041; Treatment: F1,116 = 2.065, P= 0.131; Interaction: F2,116 = 3.623, P= 0.03) Interestingly there is an interaction of BP treatment and bees: the high dose of BP causes the MA bees to consume much less pollen in the cages and have significantly lower rectum weights than the MA control and MA bees exposed to a low BP dose. The MA high dose BP treated bees also have much less vitellogenin, an important egg-yolk precursor protein, in their hemolymph. This suggests that bees that have been primed to BP by experiencing a natural hive environment may be sensitized to the BP odor and thus require larger doses of BP to modify their behavior.
34. Villa, J.D. – SELECTION FOR AN EFFECT OF HONEY BEE BROOD ON THE REPRODUCTION OF VARROA MITES – Brood from some genetic sources reduces Varroa mite fecundity (e.g. Africanized bees: Camazine, 1986 Ann. Ent. Soc. Amer. 79: 801-803); SMR/VSH bees: Ibrahim & Spivak, 2006 Apidologie 37: 31-40). This suggests a genetic component to mite fecundity which may be enhanced through selection. Mite reproduction was measured in tests of 6-12 colonies each. Each queen was caged on an empty comb for 48 h. Three days later, combs with eggs and newly emerged larvae from each source colony were moved to a common nurse colony. When the oldest larvae were one day from capping, combs were placed in an infested colony (> 0.1 mite/adult bee). Five days after, brood was moved to an incubator to develop without interference from hygienic bees for four to five days. Pupae were then examined for foundress females, progeny mites, developmental stage of pupae and anomalies in mite development. Only colonies with more than 28 infested cells were used in analyses.
In 2008, 23 colonies from five genetic sources were screened to select potential parents. In 2009, two sequential generations of colonies were propagated from colonies in the previous generations identified as having high (H) or low (L) mite reproduction. The colonies of different origins screened in 2008 varied in mite reproduction (1.97-3.46 progeny per foundress female) allowing the selection of L and H parents (Table). In 2009, L and H colonies from the first selected generation (produced using queens instrumentally inseminated with mixed semen) and also from the second selected generation (produced with single drone inseminations) differed in mean mite fecundity. These results indicate progress in selection towards a trait that may be economically useful.
Table – Mean [range] (n colonies) for the number of progeny per foundress female in infested pupal cells from L and H colonies selected as parents and representing two generations of selection. Probability of differences between H and L colonies derived from analyses of variance in each generation are indicated.
|
Generation |
L Colonies |
H Colonies |
P>F |
|
Selected Parental (natural matings) |
2.21 [2.04 – 2.49] (3) |
3.44 [3.42 – 3.46] (2) |
|
|
First Generation (multiple drone i.i.) |
2.70 [2.30 – 3.27] (9) |
3.08 [2.65 – 3.39] (4) |
0.0002 |
|
Second Generation (single drone i.i.) |
2.13 [1.73 – 2.49] (4) |
2.98 [2.70 – 3.36] (3) |
<0.0001 |
35. Webster, T.C., F.E. Dowell, E.B. Maghirang& E.M. Thacker- DOES VISIBLE / NEAR-INFRARED SPECTROSCOPY DETECT HONEY BEE CHARACTERISTICS? – Studies of insect tissue have shown near-infrared spectroscopy to be effective in determining the sex, species, or parasitism of insects (e.g. Dowell et al., 2005 Bull. Entomol. Res. 95:249-257). Application of this method for honey bee tissues might allow rapid and non-invasive evaluations of selected organs and tissues. The scan takes only several seconds and requires that a bee is briefly immobilized by chilling or carbon dioxide. We analyzed the abdomens of honey bee queens, semen from drone bees, the heads of worker bees, and the midguts of worker bees by visible and near-infrared spectroscopy. Mated honey bee queens could be distinguished from virgin queens by their absorption spectra with 100% accuracy (Webster et al., 2009 Apidologie 40:565-569). Spectra of semen contained in glass microcapillary tubes were compared to spectra of the queens. This comparison showed that classifications of queens were likely influenced by the presence or absence of semen in the queen spermathecum. However, physiological or morphological changes that occur in the queens after mating probably influenced the classifications also.
The heads of worker bees taken from the brood nest of a hive had absorption spectra that differed from those of flying workers taken from the hive entrance. These spectra could be used to predict whether bees were from the brood nest or were collected as flying bees with 85% accuracy. However, the weights of hypopharyngeal glands taken from those worker bees were not correlated with the spectra. Apparently, physiological changes in the heads of worker bees as they transition from brood nest to flight behavior contribute to the visible / near-infrared spectra. Nosema apis infection in worker bee midguts was not correlated with the absorption spectra of those midguts compared to uninfected midguts. Continuing work will include Nosema ceranae-infected midguts. Improvements in techniques may allow this and related techniques to be more useful for analyses of these honey bee organs.
36. Williams, G.R., D. Shutler, C.M. Little, K.L. Burgher-MacLellan & R.E.L. Rogers, – NOSEMA CERANAE IN NORTH AMERICA: NASTY, NICE, OR NEITHER? WATCH VIDEO – A variety of emerging pathogens threaten western honey bees (Apis mellifera), including the microsporidian Nosema ceranae. Since jumping from the Asian (Apis cerana) to the western honey bee in the latter half of the 20th century (Chen et al., 2008 J. Invertebr. Pathol. 97:186-188), N. ceranae has become distributed nearly globally and has displaced western honey bees’ historical Nosema parasite, Nosema apis, in many regions (Klee et al., 2007 J. Invertebr. Pathol. 96:1-10).
Very little is known about the epizootiology and control of N. ceranae in western honey bees because of its recent detection. Infection resulted in sudden collapse of apparently asymptomatic colonies in Spain (Higes et al., 2008 Environ. Microbiol. 10:2659-2669), whereas multiple stressors, rather than N. ceranae alone, are implicated as the cause of Colony Collapse Disorder in the United States (van Engelsdorp et al., 2009 PLoS One 4: e6481-e6497). Numerous control methods exist for N. apis in western honey bees, including fumigation, heat treatment, and replacement of aging equipment (Fries, 1992 Bee World 74:5-19); however, most common in North America is oral administration of the antibiotic Fumagilin-B® with sugar syrup in fall and spring. In our preliminary investigation (Williams et al., 2008 J. Invertebr. Pathol. 99:342-344), Fumagilin-B® applied in the fall at the recommended dosage for N. apis controlled N. ceranae for at least 6 months; however, we did not account for potential variation among beekeeping operations.
Here we investigated effects of Fumagilin-B® on N. ceranae and on western honey bee colony strength (i.e., size of adult worker population, and amount of capped and uncapped brood, honey, and pollen) using colonies over-wintered in a single apiary, as well as investigated effects of N. ceranae on colony mortality. We also repeated our previous study using colonies over-wintered by seven different beekeepers to incorporate variation arising from beekeeping practices.
N. ceranae did not affect colony winter mortality (all P > 0.56) or any colony strength measure (all Ps > 0.17). There are a number of possible explanations for this, including false recovery of colonies (Higes et al., 2008 Environ. Microbiol. 10:2659-2669), sub-damaging N. ceranae intensities, or non-significant differences in N. ceranae intensity between treatment groups for colonies whose strength was measured (i.e., P = 0.07). Similar to our previous study, fall Fumagilin-B® treatment generally lowered N. ceranae the following spring (all Ps ≤ 0.05), although results were highly variable both within and among beekeepers. Differences in N. ceranae intensities and fumagillin effectiveness among beekeeping operations could be due to a number of factors, including beekeeper management and weather.
Understanding factors influencing colony-level pathology of N. ceranae is crucial, particularly due to conflicting data on pathogenicity of the parasite. Significant recent increases in honey bee colony losses globally are likely caused by multiple factors, including recently-introduced parasites, nutritional deficiencies, and exposure to pesticides intrinsic and extrinsic to beekeeping. Until influences of all important stressors are known, both singly and in combination, it may be difficult to understand differences in strength and mortality among western honey bee colonies.
37. Wilson, M.E., J. Skinner, K.S. Delaplane & J. Pettis – BEE HEALTH @ EXTENSION.ORG: A WEB PLATFORM FOR THTE CREATION AND DISSEMINATION OF SCIENCE-BASED RECOMMENDATIONS– WATCH VIDEO – Members of the bee research and extension community are using a new toolkit and strategy in the outreach component of research and extension projects, which includes a website at www.extension.org/bee_health. This website is part of the larger eXtension.org non-profit organization, created out of the desire to transform the Cooperative Extension System through information technology. Bee research and extension personnel became involved with this initiative out of the need to deliver objective, research-based, and credible information to the general public. Two grant programs are responsible to create a “Community of Practice” and deliver specific objective outcomes from their research programs. The USDA-ARS Areawide Program and the Managed Pollinator CAP have pooled resources to create the “Bee Health” community, while many other research and extension professionals have joined the initiative. Currently there are 65 members who specialize in honey bees and native bees. Members from state and federal programs are encouraged to integrate eXtension.org into future grant programs to make this effort sustainable and to build a long lasting knowledge base about bees. Organizational structures such as the American Association of Professional of Apiculturists, USDA-ARS bee labs, the NC1173 (formerly NC508), and the Apiary Inspectors of America have been integral in conducting research around bee decline. Organizing outreach programs under the umbrella of eXtension.org continues this efficient, collaborative strategy into knowledge delivery.
Numerous tools are offered by eXtension.org to their communities, including integration of the site within Google Analytics. This offers the ability to track which pages are the most successful and better focus our efforts to increase access to our information. Tools outside of eXtension.org are used by the community to make sure content is more accessible by the public, such as the new YouTube Channel for Bee Health at www.youtube.com/BeeHealth. Other features on the main site include news, events, and over 114 articles such as: monthly updates from the Managed Pollinator CAP, other research updates, and topics in honey bee anatomy, biology, and disease. FAQs address 120 common questions about bees. These products are an excellent start to the initiative and speak well for the potential that this project has for making accurate knowledge more accessible and sustainable practices for bee management more feasible.
38. Wu, J.Y., C. Anelli & W.S. Sheppard – SUB-LETHAL EFFECTS OF PESTICIDE RESIDUES IN BROOD COMB ON WORKER HONEY BEES – The European honey bee, Apis mellifera L., is an important pollinator for many agricultural crops. The United States Department of Agriculture estimates one-third of our diet comes from insect-pollinated plants. Of that, 80% are pollinated by honey bees (Thapa, 2006 J. Inst. Agric. Anim. Sci. 27:1-23). Unfortunately, honey bees are faced with many challenges including mites, microsporidia, viruses, poor nutrition and exposure to pesticides from in-hive and external sources.
Various miticides have been used since the late 1980’s in the U.S. to treat the external parasitic mite Varroa destructor Anderson & Trueman (Varroidae) in honey bee colonies. Honey bees are often exposed to high and frequent doses of miticides as beekeepers seek to counter the mite’s rapidly developing resistance to chemical treatments (Elzen et al., 1999 Apidologie 30:17-19; Elzen et al., 2000 Apidologie 31:437-441; Pettis, 2004 Apidologie 35:91-92). Exposure to multiple pesticides is likely to occur during flight and foraging activities (Rortais et al., 2005 Apidologie 36:71-83). Returning foragers may further contaminate nest-mates, comb, and food resources. Current risk assessments for pesticides typically examine lethality of adult bees to a single active ingredient in a laboratory setting 24 or 48 hours after exposure. However, sub-lethal effects of pesticide residues on immature bees and effects from pesticide mixtures are not routinely used to assess the toxicity of pesticides during the registration process.
This study investigates pesticide exposure that honey bees face by exposure to the pesticide load or concentration of residues found in brood comb. We further investigated sub-lethal effects of pesticide residues in brood comb on larval survivability, adult longevity, and susceptibility to other pests and pathogens, such as Varroa destructor mites and Nosema ceranae (Nosematidae) spores. Worker bees were reared in either contaminated comb, containing high levels of pesticide residues, or in relatively uncontaminated “clean” comb within the same colony. Comparisons of survivability from egg to adult emergence and larval development rates yielded lower survivability and evidence of delayed development for bees reared in highly contaminated combs. Newly emerged adult bees reared from contaminated combs lived, on average, four days less than control bees in cage longevity tests. Preliminary results indicate no difference in susceptibility to Varroa mites. However, in a separate field experiment, a significantly higher proportion of bees reared in contaminated comb were infected with Nosema ceranae spores at a younger age and with higher infection levels than bees reared in clean comb. This suggests early exposure to pesticide residues during development can have serious effects on larval survivability and subtle delayed effects in the adult stage.
For Addresses of authors and footnotes, see the .pdf of the 2010 ABRC proceedings.
Videos of conference presentations
- ABRC2010 A New Assay to Measure Mite Grooming Behavior
- ABRC2010 Pesticide in Pollen Trapped from Honey Bee Hives in Connecticut
- ABRC2010 A Test for Sub-acute Effects of Some Commonly Used Bee Hive Chemicals
- ABRC2010 Pests Pathogens and Queen Losses in Commercial Migratory Operations
- ABRC2010 Apis mellifera Proteomics of Innate Resistance
- ABRC2010 Variability and Correlations Among Five Traits Associated with American Foulbrood Resistance in a Canadian Breeding Population
- ABRC2010 Effects of a Miticide on Honeybee Memory – is the Cure Worse than the Disease
- ABRC2010 Nosema ceranae in North America Nasty Nice or Neither
- ABRC2010 Beneficial Microflora in Honey Bee Colonies
- ABRC2010 Molecular Diagnostics of Nosema ceranae and apis from Honey Bees in New York
- ABRC2010 Nestmate Recognition in the Honey Bee Apis mellifera
- ABRC2010 Effects of Varroa Mites and Bee Diseases on Pollination Efficacy of Honey Bees
- ABRC2010 Effect of Transportation on Honey Bee Physiology
- ABRC2010 Disinfection of Nosema ceranae Infected Comb by Irradiation Acetic Acid and Heat
- ABRC2010 Interactions Between Nosema Microspores and a Neonicotinoid on Honey Bees
- ABRC2010 Pesticide Analysis at the Stationary Apiaries
- ABRC2010 Bee Health at eXtension
- ABRC2010 Drug Interactions Between In-hive Miticides and Fungicides in Honey Bees
- ABRC2010 Bee CAP Overview and Stationary Apiaries
- ABRC2010 Preference of Phoretic Mites for Young Bees Increase Varroa Fitness
- Varroa Sensitive Hygiene and Mite Reproduction
- ABRC2010 Safe Exchange of Honey Bee Germplasm
Source:
June, 2010 issue of The American Bee Journal, Dadant