The 2011 American Bee Research Conference was held January 6-7 at the San Luis Resort in Galveston, TX. The twenty fifth American Bee Research Conference will be held in Beltsville, MD in conjunction with the annual meeting of the Apiary Inspectors of America in February 2012. The following are abstracts from the 2011 Conference.
- Published in the May 2011 issue of the American Bee Journal. Download .pdf of the Proceedings of the American Bee Research Conference 2011 here.
1. Arechavaleta-Velasco, M.E., K. Alcala-Escamilla, C. Robles-Rios & G.J. Hunt – IDENTIFYING CANDIDATE GENES FOR HONEY BEE MITE-GROOMING BEHAVIOR USING FINE-SCALE MAPPING
Varroa-sensitive hygienic behavior and mite-grooming behavior have been identified as perhaps the most important traits that reduce Varroa mite populations. In this study, honey bee mite-grooming behavior was evaluated with a laboratory assay. Worker bees taken from the brood nest were chilled briefly at 4 °C until almost immobile and then a mite was placed on the thorax. The bee with the mite was placed in an arena that contained wax-coated foundation, food and nestmates. The time that elapsed before the bee engaged in grooming behavior (swiping at the mite with her legs) was recorded. After testing samples of bees from many colonies, a cross was made between a high mite-grooming colony and a low mite-grooming colony. A hybrid (F1) daughter queen was raised and backcrossed to a drone from a high mite-grooming colony to produce a family of workers to be used for mapping quantitative trait loci (QTL) that affect this trait and to search for the genes involved. The cDNA of the F1 queen was sequenced to identify single-nucleotide polymorphisms (SNPs) in DNA that would differ among her offspring. Probes were designed for these SNPs that could be used on Illumina Bead Station genotyping arrays. Four hundred backcross workers were tested for their grooming behavior prior to extracting their DNA. DNA samples from a subset of 96 workers were analyzed with 1,536 SNP probes within gene sequences that cover the entire honey bee genome on the bead station. MapQTL software and a map of 1,348 informative SNP loci were used to associate the grooming behavior of the bees with their genotypes. The genotypes of 15 workers indicated that they were drifters that were unrelated to the mapping family, so they were excluded from further analyses. The log of the time elapsed before responding to mites was used as a trait for interval mapping of genes that influence this trait. A putative QTL on chromosome 5 was identified (the likelihood test statistic was LOD 2.37). A second suggestive QTL (LOD 1.94) on chromosome 4 was found. The location of this QTL corresponded with a QTL for Varroa-sensitive hygienic behavior (VSH) identified in another study (see abstract 32 of these Proceedings), indicating that one or more genes may influence both of these mite-resistance traits. Finally, a third putative QTL (LOD 1.92) was found one chromosome 10. The genes that lie in these QTL regions are currently being studied for their possible functions, and more individuals are being added to the analyses to increase the precision of mapping. It is particularly interesting that one region may influence both mite-grooming behavior and VSH. These studies should help future development of bees that have high tendencies for both traits that give them the ability to remove Varroa mites from the hive.
2. Aronstein, K., R. Cox, E. Saldivar & T.C. Webster – COMPARATIVE STUDIES OF TWO NOSEMA SPECIES IN HONEY BEES
The objective of this research was to determine differences in the effects of two species of Nosema on honey bee health. A recently detected species, N. ceranae, has been reported to be more virulent than N. apis, the original species in the Western Hemisphere. N. ceranae has also been implicated as a possible cause of “colony collapse disorder” (CCD).
In a series of four experiments, laboratory cages of 100 worker bees were fed one of two species of Nosema spores or a 50:50 mixture of the two species. Dead bees were counted and collected each day for later analysis. We examined the bee gut contents microscopically to determine the percentage of bees infected or to calculate the number of Nosema spores per bee. Nosema inoculated bees became infected and produced millions of spores/bee within 3-5 weeks.
In most experiments we also found that N. ceranae was not more virulent than N. apis in laboratory cages. Actually, N. apis inoculated bees seemed to die sooner, and a greater percentage of the bees were infected when compared to N. ceranae inoculated bees. When inoculated with a high dose of the mixture of the two species (50,000 spores – 25,000 of each species), bee mortality was about the same as with N. apis inoculated bees. In four experiments, infection with N. apis always resulted in higher bee mortality three or more weeks post inoculation than with N. ceranae. In these same experiments, N. apis spores caused a greater percentage of the bees to become infected than did N. ceranae when bees were inoculated with higher doses of Nosema spores (>5,000). In the fourth experiment, bees inoculated with 500 spores of N. apis died sooner than bees inoculated with the same dose of N. ceranae. Further, it appears that inoculation with 5,000 or more spores of either species of Nosema results in a shorter life span for worker honey bees.
Do these laboratory results apply to field colonies of honey bees? A small scale field study planned for the spring of 2011 may yield results to help answer this question. If these results do apply to field colonies of honey bees, then colony health is at no greater risk from N. ceranae than from N. apis. Actually, N. apis may shorten the life of worker bees more than N. ceranae at lower doses. Additionally, the results of these experiments do not support the hypothesis that the newly introduced species of Nosema is the primary cause of CCD.
3. Aronstein, K., F. Drummond, B. Eitzer, J. Ellis, J. Evans, N. Ostiguy, S. Sheppard, M. Spivak & K. Visscher – THE CAP STATIONARY APIARY PROJECT: COLONY STRENGTH DATA ANALYSIS 2009-2010
A project was initiated in 2009 to assess causal factors for colony loss in stationary apiaries in seven states across the U.S. Thirty colonies were started from packages in Minnesota, Maine, Pennsylvania, Texas, Washington, and Florida in 2009. Two other apiaries were started in 2010 in California and Maine (n= 15 colonies). All packages were re-queened with Italian queens in April or May in the starting year of each apiary. Monthly sampling of colonies was conducted to assess colony strength (brood and workers), queen status and all pests and pathogen symptoms. In addition, collections of workers were made for dissection assessment of Nosema spp. infestation, tracheal mite infestation and molecular determination of virus infections. Viruses screened included Deformed Wing Virus (DWV), Black Queen Cell Virus (BQCV), Israeli Acute Paralysis Virus (IAPV) and Sacbrood Virus (SBV). We found that the pattern of colony loss was significantly different across sites. Abiotic factors that appear to be related to the level of loss and/or supercedure were maximum temperature during the brood rearing season, the percent of agricultural land within a 2 mile radius of the apiary, and pesticide contamination of pollen. Biotic factors were used to develop statistical models for estimating risk of colony loss. Initial models suggest that Nosema infection, Varroa mite infestation and IAPV infection are all significant causal factors of colony loss, but there were significant apiary site interactions with some of these factors. This project will be continued for another 1-2 years.
4. Chaimanee, V., Y. Chen, J. Pettis & P. Chantawannakul – Nosema SPP. IN EUROPEAN AND NATIVE HONEYBEES IN NORTHERN THAILAND
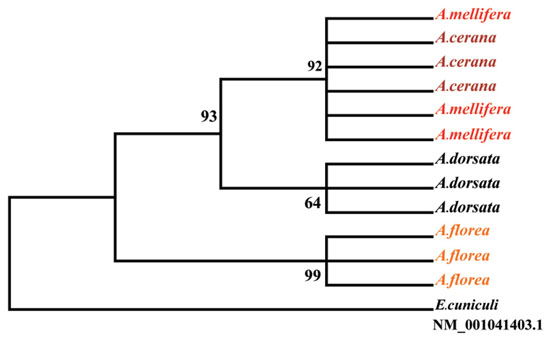
The microsporidium Nosema ceranae was detected in honey bees in Thailand for the first time. In this study, we collected and identified species of microsporidia from the European honey bee (Apis mellifera), the cavity nesting Asian honey bee (Apis cerana), the dwarf Asian honey bee (Apis florea) and the giant Asian honey bee (Apis dorsata) from colonies in Northern Thailand. We used multiplex PCR technique with two pairs of primers to differentiate N. ceranae from N. apis (Hernández et al., 2007 Appl. Environ. Microbiol. 73: 6331–6338). From 80 A. mellifera samples, 62 (77.5%) were positive for N. ceranae. Amongst 46 feral colonies of Asian honey bees (A. cerana, A. florea and A. dorsata) examined for Nosema infections, only N. ceranae could be detected. No N. apis was found in any of our samples of the four Apis species (Chaimanee et al., 2010 J. Invertebr. Pathol. 105: 207–210).
If we consider the phylogenetic relationships between N. ceranae isolates and the nesting behavior of the host Apis species, an interesting pattern was evident. Based on the partial sequences of polar tube protein 1 gene using maximum parsimony, the data showed that N. ceranae isolated from A. mellifera grouped into the same clade as the N. ceranae that isolated from A. cerana supported by a high bootstrap value (92%) (Figure), both of which nest in cavities and build multiple combs. The single comb nesting species, A. dorsata and A. florea, each harbored distinct isolates of N. ceranae. Our data indicate that some degree of specialization or isolate variation is occurring as N. ceranae invades Apis species in South East Asia.
5. Dahlgren, L.P., R.M. Johnson, M.D. Ellis & B.D. Siegfried – VARROACIDE TOXICITY TO HONEY BEE QUEENS
As a honey bee queen is the colony’s sole egg layer, the effects of a toxin on the queen can be more important to colony success than the toxin’s effects on her worker offspring. We established a dose-response curve for queen honey bees exposed to varroacides commonly used in honey bee colonies. Five hundred and seventeen queens were raised to find the LD50 for tau-fluvalinate, coumaphos, amitraz, thymol, and fenpyroximate. Sister virgin queens were grafted from a single source colony. Virgin queens were then emerged in queen banks, and when 4 days old, they were removed for 1 h to complete varroacide treatment. There were three or more replications for each varroacide. Each replication had at least 15 virgin queens that were topically treated with a range of doses including doses expected to cause 0% and 100% mortality. Mortality was checked at 24 h, 48 h, and then at 1 week intervals for 6 weeks.
We found that queens are much less susceptible than workers to tau-fluvalinate, coumaphos, fenpyroximate and thymol. There were no significant differences between worker and queen mortality when treated with amitraz. Coumaphos and fenpyroximate did not produce reliable queen mortality at the highest dose tested, 500 µg and 300 µg respectively (Table); consequently, we could not establish a precise LD50 and minimum possible LD50s are reported. Future studies will examine the basis for the high tolerance of queens to some varroacides. Mortality is not the only way varroacides can affect queens, and our findings provide a foundation for future studies of queen fecundity, egg-laying rate, brood survival, pheromone output, retinue formation, and behavior.
6. Eischen, F.A., R.H. Graham, R. Rivera & R. James – CONTROLLING NOSEMA SPORES ON STORED HONEYCOMB
In previous work, we had observed significant numbers of living Nosema ceranae spores on stored honeycomb. In this study we tested five fumigants, viz., methyl bromide, phosphine (Phostoxin), formic acid, ethylene oxide, and ozone for efficacy in controlling Nosema ceranae spores on stored honeycomb. Standard Langstroth-sized honeycombs harboring living spores were exposed to a dose range of fumigants. They were then tested for survival of spores using sytox green and DAPI stains or placed into nucleus colonies having no detectable levels of Nosema. Colonies receiving treated combs were checked for Nosema infection on days 0, 12 and 38.
In general, phosphine (Phostoxin) at the recommended label dosage (45 tablets/28.4m3) was the most successful. Less than 10% of spores showed viability with Sytox green and DAPI stains. This efficacy was confirmed when treated combs were placed in uninfected colonies (Table). After 38 days of exposure, we found 40,000 spores/bee. This was significantly lower than the 7.65 million spores/bee in colonies receiving an untreated comb (P < 0.05).
Both methyl bromide and formic acid at their respective label doses caused small, but significant declines in viable spore numbers. This was confirmed when treated combs were placed in “Nosema-free” colonies (Table). In the nucleus test, no significant differences were detected among the three compounds; however, we suspect that small sample size (n = 6) prevented this. Limited testing with ethylene oxide found that control was excellent, but is expensive. Tests with ozone thus far have proved inconclusive. Since Phostoxin is labeled for protecting stored combs against wax moth, we see it as a good choice for controlling N. ceranae on stored combs.
7. Eischen, F.A., R.H. Graham & R. Rivera – IMPACT OF NUTRITION, VARROA DESTRUCTOR AND NOSEMA CERANAE ON COLONIES IN SOUTHERN LOUISIANA
This study examined the impact of three factors, i.e. nutrition, Varroa, and Nosema acting singly or in combination on colonies during a 14 month period (Oct. 2009 – Dec. 2010) in southern Louisiana. Colonies (n = 50) were randomly assigned to eight treatment groups: 1. negative control (no diet, no medications); 2. diet, no medications; 3. no diet, fumagillin; 4. diet, fumagillin; 5. no diet, amitraz; 6. diet, amitraz; 7. no diet, fumagillin, amitraz; 8. diet, fumagillin, amitraz. Starting in early October 2009, colonies receiving diet were fed either 454g or 908g of Bee Pro + 4% Prolen (Mann Lake Ltd.) about every 10 days. Medications were applied at the start of the trial and amitraz again after five and nine months.
Colonies were moved to California (Jan. 2010) for almond pollination and feeding stopped. At this time strength levels were highest for all groups receiving diet. Those colonies that received diet and both medications were largest. Regardless of treatment group, by late January 2010 colonies that started the trial with 149 Varroa or less (natural mite drop during 72hrs) had a significantly greater percentage of colonies that met a minimum 6-frame strength criterion suitable for almond pollination than colonies that had 150 mites drop. Colonies with 150+ Varroa were slightly larger at the start of the trial, but exhibited a marked decline in strength. Colonies treated for Varroa had a significantly higher percentage of colonies meeting a 6-frame strength criterion than those left untreated. However, treating for Varroa in colonies that had 150+ Varroa, did not develop into as large of colonies as those with <150 mites.
Colonies returned to Louisiana in late March and were supered. Honey production was measured in late August and was, on average, about 79kg for colonies receiving both medications. Those treated for Nosema or Varroa only produced about 55 and 47kg, respectively. Control colonies produced about 34kg of honey.
Survival was greatest for colonies receiving diet and both medications (66%). Survival varied between 34 – 46% for the other seven groups. Adult strength was greatest for the group receiving both medications (14.45 frames). All groups receiving fumagillin were significantly larger than those that were only treated for Varroa or left untreated. Untreated colonies and those receiving diet only were, on average, either 3.2 or 1.9 frames of bees, respectively in December 2010.
We conclude that good nutrition, Varroa and Nosema control are essential for colony performance especially if colonies will be used for almond pollination or honey production.
8. Ferrari, T.E. & A.B. Cobb – CORRELATIONS BETWEEN GEOMAGNETIC STORMS AND COLONY COLLAPSE DISORDER
In 1994, the National Oceanographic and Atmospheric Administration (NOAA) and the Solar Weather Prediction Center (SWPC) began recording disturbances to Earth’s magnetosphere caused by the sun’s solar eruptions. Separately, three independent groups of scientists surveyed honey bee losses from August thru February. Our review of their data indicates that a perfect bivariate correlation exists between severe geomagnetic storms and CCD loss estimates.
First: Camazine et al. (Entomology Department, Penn State University, Newsletter No. 77) reported two statewide surveys assessing colony losses by beekeepers during fall/winter periods from August to March, 1995/1996 and 1996/1997. Independently, SWPC/NOAA recorded geomagnetic activity during the same periods. After the 1995/1996 winter, beekeepers reported 51% colony losses, and SWPC/NOAA reported 21 hours of major geomagnetic storms. During the 2006/2007 period, colony losses declined to 26%; concomitantly, major storm durations declined to 12 hours. Over two-consecutive fall/winter periods, honey bee losses declined 51% and geomagnetic storms declined 57%. This parallel would normally be considered a happenstance (P=0.25) were it not for two subsequent surveys.
Second: Burdick and Caron (2006, MAAREC Beekeeper Survey. http://maarec.cas.psu.edu/pdfs/MAARECSurveyPRELIMAA.pdf) reported survey results conducted in the Northeast during six fall/winter periods from 2000 to 2006. Apiary inspectors reported colony losses in Maryland, Delaware, Pennsylvania, New York and New Jersey. Information requested was what beekeepers’ total colony losses were. Thus, CCD was only one component amongst all causes for colony failures. Simultaneously, SWPC/NOAA recorded geomagnetic activity.
As major storm durations escalated, corresponding increases in colony losses occurred and weak, positive correlations emerged. Considering the variance imposed because multiple causes of colony losses were reported and different numbers of states were surveyed from year-to-year, a relatively weak correlation was not surprising. Of statistical significance, however, is that when correlations of colony losses with progressively greater storms were compared, a graph of respective correlation coefficients was nearly perfect (R2=0.978). Thus, evidence indicates that as geomagnetic storm intensities increased — while all colony loss causes remained constant — correlations with colony losses became stronger.
Third: During three fall/winter periods, from August to March 2006 to 2009, apiary inspectors surveyed colony losses throughout the United States (van Engelsdorp et al., www.plosone.org/article/info:doi/10.1371/journal.pone.0004071). Beekeepers were specifically asked to estimate “what percent of their total losses had no dead bees in the hive,” a key CCD symptom. Again, SWPC/NOAA independently recorded Earth’s geomagnetic activity. Results were consistent with the two prior surveys: graphs of major storm durations and colony losses due to CCD produced a perfect positive correlation (R2=1.0) between the two independent variables. Data represent a larger database and greater area than the MAARC survey; a more consistent number of states were sampled, and, importantly, the survey targeted CCD losses.
Numerous biomagnetic studies indicate that orientation behaviors of many organisms are altered by imposed magnetic fields or anomalies. During three different time periods, totaling 11 years, as major geomagnetic storm durations increased there was a systematic increase in colony losses. Foragers contain superparamagnetic magnetite, providing a basis for magnetoreception. In conclusion, surveys and experimental evidence indicates that foragers possess a magnetoreception sense for orientation purposes and that over long distances major geomagnetic storms can interfere with their homing ability, causing them to get lost and “disappear.”
9. Ferrari, T.E. & A.B. Cobb – HONEY BEES, MAGNETORECEPTION AND COLONY COLLAPSE DISORDER
CCD has afflicted honey bees for decades, if not centuries. The disorder predates most pesticides, diseases, pests and management protocols. Scientists have searched – unsuccessfully – for chemical and biotic causes for this scourge.
Behavioral scientists have established that many organisms extract directional information from Earth’s ambient magnetosphere. Anomalous magnetic fields have also been shown to alter a honey bee’s orientation behavior (Winklhofer, M.J., 2010 J. Royal Soc. Interface 7: S131-S134; Johnson, S. and Lohmann, K.J. 2008, Physics Today 61: 29-35). Their “magnetoreception” sense is controlled by magnetite (Fe3O4) in adult bee abdomens (Hsu and Li, 1993 J. Exper. Biol. 180: 1-13). Linked together, magnetite crystals act like a compass needle and orient bees in magnetic fields. Discovery of magnetoreception in foragers resulted in astonishing interrelationships between the sun’s explosive eruptions, geomagnetic perturbations they produce to Earth’s magnetosphere and why they disrupt a bee’s homing ability. Our theory is that solar storms interfere with Earth’s magnetic fields, a forager’s perception of it and consequently it’s homing ability.
CCD’s epidemiology, symptoms and involvement with a forager’s magnetoreception sense are interrelated. Considering a bees’ behavior, our theory makes shrewd predictions regarding how major geomagnetic storms reveal CCD’s six key symptoms (Wilson, W.T. & D. M. Menapace, 1979 Am. Bee J. 119: 184-186, 217). Geomagnetic storms interact with a honey bee’s magnetoreception sense depending on what developmental stage they are at and what they are doing when it occurs:
Spring, Summer, Fall: Only foragers contain magnetite and, therefore, are vulnerable to a major storm. Disoriented and without accurate coordinates to return to their hive, they fly away and get lost. Unlike diseases, there would be no evidence of their demise — CCD’s leading symptom.
If a queen is exposed to a storm during a mating flight, that could lead to disaster. Fortunately, long distance mating flights are unlikely and visual references would suffice to lead a queen safely back to the hive. Hence, healthy egg-laying queens are present in colonies suffering from CCD — a second symptom.
Summer: Larvae, pupae and young bees are insensitive to fluctuations in the magnetosphere because they don’t contain magnetite. Bees accumulate magnetite beginning a few days after emerging from cells. After a storm, young bees can replace lost foragers and save a colony. This is likely to occur when summer brood rearing peaks. That colonies suffering from CCD have brood and young adults present are common observations — a third symptom.
Fall: Geomagnetic storms appear dreadfully deadly during fall because forager losses would reduce pre-winter food collection and produce weak winter clusters — a fourth symptom.
Winter: With limited food and insufficient bees to maintain a habitable cluster temperature during winter, a fall storm would render colonies susceptible to cold. Sadly, a storm’s impact might not surface until spring when frail or lifeless colonies become noticed — a fifth symptom.
A sixth observation is that CCD is not contagious: equipment from affected hives can be used on healthy colonies without causing ill effects. If such colonies do decline, the original loss was likely due to disease and was misdiagnosed as CCD.
10. Frazier, J.L., M.T. Frazier, C.A. Mullin & W. Zhu – DOES THE REPRODUCTIVE GROUND PLAN HYPOTHESIS OFFER A MECHANISTIC BASIS FOR UNDERSTANDING DECLINING HONEY BEE HEALTH?
The reproductive ground plan hypothesis links a pleotropic set of genes for reproductive anatomy (ovary size), reproductive physiology (yolk protein level), sensory physiology (sucrose sensitivity), and foraging behavior (pollen/nectar). While the evolutionary significance of ground plan genes is open to debate, substantial experimental evidence indicates that the above factors co-vary, can be selected for at the colony level, and that down regulation of specific genes affects more than one of these components. Multiple environmental factors including nutrition, mite feeding, pesticides, and colony manipulations are known to alter the expression of these components involving a common set of hormonal regulatory pathways. Given a specific set of genes and some common regulatory pathways, specific mechanism based hypothesis testing may uncover new relationships for the actions of factors known to impact honey bee health and colony dynamics. Specific examples of environmental factors altering the expression of these ground plan components will be presented. Testable hypotheses of the potential actions of pesticides and the benefits of combining these with simulation modeling to determine their impacts on honey bee population dynamics will be discussed.
11. Frazier, M.T., S. Ashcraft, W. Zhu & J. Frazier – ASSESSING THE REDUCTION OF FIELD POPULATIONS IN HONEY BEE COLONIES POLLINATING NINE DIFFERENT CROPS
Beekeepers pollinating agriculture crops are concerned about the reduction of colony field forces and the ultimate weakening of their colonies. Pesticide exposure is a potential factor in these field force reductions. During 2009-2010 we assessed changes in the field force populations of nine colonies on each of nine crops by counting foragers leaving colonies at regular intervals during the pollination period for each crop. We also collected for pesticide analysis, dead and dying bees at the hive, returning foragers, crop flowers, trapped pollen, and corn flowers associated with the cotton crop. Field forces were significantly reduced in colonies pollinating cotton, corn and alfalfa, while those on, apples, pumpkins, almonds, melons, blueberries and wild flower honey production, increased or remained fairly consistent.
A total of 52 pesticide residues were identified in samples collected across the nine crops. In cotton, returning foragers had no pesticide residues, while dead and dying bees collected around hives had 11 residues including 306 ppb acephate. In alfalfa, returning foragers had three residues, while dead and dying bees had 10 residues including 12.7 and 59.5 ppb of thiamethoxam and esfenvalerate, respectively. Dead and dying bees collected around colonies in association with corn had only residues of 2,4-DMPF at 5,160 ppb and fluvalinate at 3.4 ppb. Overall, fungicide residues occurred frequently and often were found at high levels. Chlorothalonil was found in pumpkins in trapped pollen (1100 ppb) and dead and dying bees (2290 ppb). Captan was found in blueberries (1310 ppb), apples, (3860 ppb), and in trapped pollen. In almonds iprodione, pyraclostrobin and boscalid were found in trapped pollen at 3260, 3480 and 7270 ppb, respectively. In-hive miticides were typically found at low levels, the highest levels of fluvalinate and coumaphos were 87.6 and 18.6 ppb. The exception to this was the amitraz metabolite, 2,4-DMPF, found at 5,160 ppb in dead bees in corn. Clearly honey bees used for crop pollination are being exposed to a diverse array of agrichemicals, especially fungicides, and the impacts of this on colonies following the exposure period needs further investigation. Since honey bees are polylectic, requiring diverse sources of pollen, the establishment of flowering, pesticide-free refugia near bee-pollinated crops could help mitigate exposure to and impacts of pesticides.
12. Hood, W.M. & S. Peterson – COMPARATIVE TRAPPING INVESTIGATIONS OF SMALL HIVE BEETLES INSIDE HONEY BEE COLONIES
Three small hive beetle traps which are currently marketed in the U.S. were compared for their trapping efficiency during a full season in 2010. Field tests were conducted at Clemson University, South Carolina, using the Freeman Beetle Trap, the Better Beetle Blaster Trap, and the Hood Trap. The Freeman trap consists of a hive bottom made of wood and screen that allows beetles to freely enter a removable plastic tray (partially filled with vegetable oil) below. The Better Beetle Blaster trap (Cutts trap) is a plastic reservoir, half-filled with vegetable oil, and is designed to be fitted between two frame top bars in the hive. The Hood Trap is a small plastic box trap that is fastened inside a hive frame. The trap has three compartments, the middle compartment filled with cider vinegar as an attractant and the two side compartments half-filled with food grade mineral oil as the killing agent.
The primary objective of this research project was to compare the number of adult beetles killed in the three traps when placed in new colonies that were established from package bees. The other objective was to measure and compare other colony parameters including adult bees, capped brood, honey, and Varroa mites.
Four apiaries were setup in the Clemson University Experimental Forest. Eight test colonies were established in each apiary with 2-lbs package bees on 6 April 2010. On 3 May, colonies were randomly selected in each apiary to receive one of four treatments: Cutts Trap, Freeman Trap, Hood Trap, or no trap (control). Treatments were replicated twice in each apiary. All 32 test colonies were fitted with Freeman trap hive bottoms.
The Cutts, Freeman, and Hood traps were serviced at 2-week intervals through 2 November by removing and counting dead beetles and replenishing traps with vegetable oil, vinegar or mineral oil as appropriate. Each test colony received a 1-day survey for beetles and a 3-day Varroa mite survey at 6-weeks intervals through 19 October by placement of a clean Freeman Trap tray with fresh vegetable oil and a Varroa mite sticky board. Other colony parameters were measured at 8-week intervals through 19 October. An end of season total “colony shakeout” of beetles was conducted on all colonies on 8 November to count adult beetles remaining in colonies.
Thirteen 2-week trap counts of beetles killed were compared. Significantly more (P<0.05) beetles were killed in the Freeman traps versus the Cutts and Hood traps. There were no differences in number of beetles captured in the Cutts traps versus the Hood traps. There were no overall differences in the 1-day Freeman trap surveys. There was no significant difference (P>0.05) in the mean number of beetles counted during the total colony shakeout of beetles (Freeman/62, Cutts/75, Hood/82, and control/128). There were no overall treatment differences in the other colony parameters.
This investigation suggests that the Freeman Trap proved to be a more efficient trap based upon the number of beetles removed from the colonies. The low mean number of beetles remaining in the control colonies at the end these investigations suggests that traps in the remaining colonies may have provided “trapping sinks” in test apiaries.
13. Hopkins, B.K., C. Herr & W.S. Sheppard – PRODUCTION OF HONEY BEE (APIS MELLIFERA) QUEENS USING CRYOPRESERVED SEMEN
As primary pollinators, honey bees are an essential contributor to modern agriculture, contributing directly to the production of a third of the human diet. As such, the crisis that is causing decimation of honey bee colonies is of grave concern. In general, the health of the colony is affected by two factors, the environment and genetics. Genetic differences among colonies within populations are responsible for differences observed in parasite and pathogen resistance. There are many potentially valuable and rare alleles in the honey bee populations within and among 26 distinct subspecies and, presumably, here in the U.S. However, substantial annual colony losses, small breeding populations, selection and genetic drift all contribute to reduced genetic variation, and the loss of potentially valuable and possibly rare alleles is a concern. Increased genetic diversity even within colonies can increase fitness and productivity and reduce parasite and pathogen loads.
Cryopreservation of semen has the potential to preserve honey bee genetic diversity in the form of spermatozoa and provide breeders with a source of genetic variation that can be used to interject new alleles into a breeding program from which to select upon. The focus of our research is to develop a cryopreservation technique that will provide a valuable tool for breeders and make possible a genetic repository for global honey bee genetic diversity. Liquid nitrogen storage of germplasm is a cost effective method for long-term storage of genetics, and a stable medium for distribution that can ameliorate problems associated with the seasonality of breeding. The improvement that cryopreservation can have on breeding stock has been well demonstrated in the cattle industry. More than half of the artificial inseminations in cattle breeding utilize preserved semen and have contributed to substantial increases in milk production.
We have improved the cryopreservation of honey bee semen and demonstrated the ability to produce sequential generations of queens by backcrossing each generation to a single stock of cryopreserved semen. These results demonstrate two valuable uses for frozen honey bee semen that have not been possible before: 1) the ability to produce viable queens using cryopreserved semen, and 2) the ability to reconstruct precious subspecies through multiple generation backcrosses to the same stock of preserved semen. There are many ways in which a breeder can use cryopreserved semen in a selective breeding program. For example, with the development of marker-assisted selection tools, aliquots of frozen semen could be screened prior to use for even greater efficiency and specificity toward achieving selection goals.
14. Johnson, R.M. & M.D. Ellis – BROODMAPPER: A WEB-BASED TOOL TO MONITOR THE EFFECTS OF PESTICIDES ON HONEY BEE BROOD
Experiments looking at the effects of pesticides on honey bees have traditionally been performed on adults; however, honey bee brood may also be affected. Brood toxicity testing needs to be performed to accurately assess the risks posed by pesticide exposure. The great amount of time required to collect brood data in the field has limited research on immature honey bees. To facilitate the monitoring of responses to pesticide exposure in brood throughout its development, we developed a web-based technique for scoring series of digital photos of brood taken throughout brood development in free-flying colonies. We created a JavaScript tool that we named Broodmapper for scoring brood photographs using the open source Processing language and environment. To use Broodmapper, a hexagonal grid overlay is placed on the digital image and the operator clicks on each cell to record the cell contents. The Broodmapper program then creates a data file of cell contents for each image. To test the program we tracked brood survival in eight nucleus colonies with pairs of colonies each fed Rally™ WSP (40% myclobutanil) in sugar syrup at a dose of 0, 20, 60 or 200 mg every 4 days. Three photos of brood from each colony, with each photo containing 120 cells, were taken during 8 visits to each colony over the course of the 21-day brood development period. We used map pins to delineate the three arenas that were photographed. We then scored all photographs using the Broodmapper tool (Figure), and performed survival analysis on the data. High variability in brood survival was observed among these 8 colonies, with no relation to the level of myclobutanil exposure. These results indicate that more experimental colonies are needed to assess the effects of pesticide exposure on brood survival. Broodmapper makes it possible for students with little beekeeping experience to make significant contributions to the research enterprise, while at the same time greatly reducing the time required to collect data in the field.
15. Kirrane, M.J., L.I. de Guzman, T.E. Rinderer & P.M. Whelan – EXAMINATION ON THE REPRODUCTIVE SUCCESS OF RE-INVADING VARROA MITES
High proportions of non-reproductive (NR) Varroa mites have been reported in honey bee colonies exhibiting resistance to this parasite. This non-reproduction is associated with hygienic behavior (Harris et al., 2010 Ann. Entomol. Soc. Am. 103: 146-152); however, the mechanism by which this occurs is unknown. Two experiments were designed to assess the reproductive output of re-invading Varroa mites. The first involved the transfer of foundress Varroa from adult bees (PH) and different stages of brood into newly sealed larvae. The brood stages were: newly sealed larvae (SL), pre-pupae (PP), white-eyed (WE) and pink-eyed (PE) pupae. For experiment 2, Varroa mites were obtained from SL and inoculated into SL, PP, WE and PE cells. The transfer technique described by De Ruijter et al. (1987 Apidologie 18:321-326) was used. Reproductive mites were defined as those that produced both a mature male and a mature female offspring.
In experiment 1 reproductive success was low overall and mostly observed in the SL to SL transfer (13%, Table). Unsuccessful reproductive attempts were highest in SL to PP (61%) transfer followed by SL to SL (22%). A high proportion of Varroa mites coming from WE (83%), PH (89%), and PE (92%) did not produce any progeny. Similar trends were observed in Experiment 2. Successful reproduction only occurred in the transfers to SL (26%). Generally, lack of progeny was not associated with mites having a lack of stored sperm.
Our results suggest that mites removed from pre-pupae or older brood cells are unlikely to produce viable daughters. Similarly, if mites enter brood stages other than SL they will likely be non-reproductive. Hence, the ability of honey bees to remove Varroa-infested brood will enhance the pool of NR mites and negatively affect mite population growth in hygienic colonies.
16. Krupke, C., B. Eitzer & G.J. Hunt – POTENTIAL ROUTES OF EXPOSURE TO HONEY BEES FROM NEONICOTINOID CORN SEED TREATMENTS
Reports from several beekeepers of apparent bee poisonings at the time of corn planting in Indiana in 2010 led us to investigate whether neo-nicotinoid seed treatments could be involved. Samples were analyzed at the Connecticut Agricultural Experiment Station by LC-MS-MS optimized for pesticide analyses. Dead and dying bees from 5 hives in three apiaries had 3.4 to 7.6 ppb of clothianidin. Pollen collected in one day by the foragers of a colony showing poisoning symptoms had about 20 ppb of each of the two neo-nicotinoids (clothianidin and thiamethoxam) commonly used to treat corn kernels. No neo-nicotinoids were detected in bees or bee-collected pollen from a healthy hive in a nearby apiary that was not experiencing bee kill.
In a follow-up study, corn was planted in two fields with or without seed treatment and colonies were placed in and around them. Samples from bee-collected pollen in the treated field ranged from 0 to 88 ppb of clothianidin. Bee pollen in the adjacent untreated field ranged from 0 to 13.1 ppb clothianidin. Anthers from corn that had seed treatment had up to 3.9 ppb clothianidin. Anthers from untreated corn had 0.3 ppb. Soil samples taken from fields that had corn two years in a row contained 3.1 to 9.6 ppb clothianidin. Soil from a field that had been planted with untreated soybean seed in 2010 had 2.1 ppb clothianidin.
These results show there are multiple avenues by which bees can become exposed to corn neo-nicotinoid seed treatments and that the compounds are persistent in the soil. More acreage is planted in corn in the U.S. than any other crop. Given the high toxicity of neo-nicotinoid corn seed treatments, further study is needed to find ways to minimize risks to pollinators.
17. Matisoff, M.A. & T.C. Webster – ANATOMICAL CHANGES IN THE MIDGUT AND PERITROPHIC MEMBRANE
The peritrophic membranes (PM) of adult worker honey bees (Apis mellifera) are secreted as a continuous delamination along the length of the midgut (MG). The PM consists of several membranous layers and granular components that protect the delicate microvilli in the midgut from damage by food particles and pathogens (Pabst, 1988 Entomol. Gen. 14: 1-10). The PM contains 4- 20 percent chitin and 40 percent protein as well as small amounts of acid mucopolysaccharides, neutral polysaccharides, mucins, hyaluronic acid, hexosamine, glucose, and glucuronic acid (Nation, 2002 Insect Physiology and Biochemistry. CRC Press). In addition to protecting the MG from damaging food particles, the PM compartmentalizes the midgut, optimizing transport (Schreiner, 1952 Z. Vergl. Physiol. 34: 278-298), and carrying immobilized enzymes that increase digestive efficiency and decrease enzyme excretion (Peters & Kalnin, 1985 Entomol. Gener. 11: 25-31). In this study, we examined the MG and PM in healthy bees and caged bees that were inoculated with high N. ceranae spore loads. We found severe deterioration of the PM 12 days after inoculation. This may be due to direct damage by germinating spores penetrating the PM, or due to indirect damage by interfering with the ability of the MG to generate the PM.
18. Meikle, W.G. & G. Mercadier – CHALLENGES FOR DEVELOPING BIOPESTICIDES AGAINST VARROA
Effective control of Varroa mites using biological control agents, such as entomopathogenic fungi, would reduce the number and amount of chemical agents used in bee hives, which would benefit both bee and human health, and would likely have few prospects for resistance by Varroa. Several research groups have identified and field-tested the fungi Metarhizium anisopliae and Beauveria bassiana, which are pathogenic to Varroa. With multiple applications of B. bassiana we observed significantly higher Varroa mite fall in treated hives compared to control during the application period, and significantly lower mite fall starting 10 d after application. However, we did not observe significantly lower phoretic mite densities between treated and control. Other research groups have also reported mixed success, albeit usually with single applications and with a different fungal species. Several questions concerning the Varroa / fungus/ honey bee interaction need to be addressed, including:
1) To what extent are bees affected by the fungus? We did not observe large losses of adult bees after application, but in that case how do adult bees protect themselves from the fungus?
2) For how long after application is the fungus effective? We usually observed spikes in mite fall 7-10 d after each application, which would be consistent with the effective duration of a single application being only 1-2 d.
3) Which part of the Varroa population is being attacked by the fungus? If Varroa in capped brood cells are protected (like brood), and phoretic mites are not being significantly affected, is the fungus attacking mainly emerged, non-phoretic mites? What is the impact on Varroa population dynamics?
4) How can we improve formulation or application method and still have an economically-viable treatment? Is there a way of increasing application half life without increasing danger to the bees themselves?
5) What are the contamination risks? Fungi might produce mycotoxins (probably at very low levels) or be mixed with other microbes during fermentation. Are there significant levels of undesirable compounds either in the formulation or in any bits of media mixed with the fungal conidia?
19. Mulholland, G.E., B.E. Traver & R.D. Fell – INFECTION VARIABILITY OF NOSEMA CERANAE WITHIN HONEY BEE COLONIES
Nosema ceranae is an obligate intracellular parasite that infects the honey bee midgut. Most studies investigating Nosema infections are performed on pooled samples of bees using a spore analysis; while this is more efficient than testing individuals, a few highly infected bees can skew the diagnosis. Furthermore, spore counting is not always reliable for detecting low-level infections.
We hypothesized that intra-colony variation of N. ceranae infection levels could impact diagnosis and utilized both spore counting and real-time PCR (qPCR) techniques to determine infection levels of N. ceranae within individual bees. Fifty bees from four hives were analyzed utilizing both spore counting and qPCR techniques. Using standard curve quantification, the number of copies of N. ceranae DNA present in individual samples was calculated. This approach was designed to illustrate the distribution of infection levels among individuals, as opposed to the overall colony level estimate obtained from pooled samples. Spore counts were also compared to qPCR-determined infection levels to analyze the reliability of spore counting as a diagnostic technique for individual bees.
The percentages of N. ceranae infections for individual bees in four different colonies were 6%, 2%, 0% and 8% for spore counts, and 82%, 56%, 16% and 18% for qPCR, respectively. A low correlation (R2 = 0.45) was found between qPCR-detected infections and spore count-detected infections; this finding indicates that spore counting may not provide an accurate indication of N. ceranae infection level. It was also concluded that analyzing samples containing multiple bees may not yield accurate representations of a colony’s overall infection level. The diagnostic implications of these findings need further study, particularly with regard to treatment recommendations for beekeepers.
20. Mullin, C.A., J.L. Frazier, M.T. Frazier & T.J. Ciarlo – A Primer on Pesticide Formulation ‘Inerts’ and Honey Bees
No single stress factor has been identified as the causal agent for honey bee colony collapse disorder. We have found 121 different pesticides and metabolites up to 214 ppm within 887 wax, pollen, honey bee and associated hive samples across the U.S. and one Canadian province. No individual pesticide level correlates with recent bee declines. High numbers of active ingredients and great qualitative and quantitative diversity of residues suggest that more generic formulation ‘inerts’ that co-occur across classes of pesticides may be involved. Formulations usually contain inerts at higher amounts than active ingredients, and these penetrating enhancers, surfactants and adjuvants can be more toxic on non-targets than the active ingredients. Inerts often enhance the pesticidal efficacy as well as inadvertent non-target effects of the active ingredient after application.
We have found that the nonionic surfactants Triton X-100 and Silwet L-77 were highly lethal to adult honey bees at an oral dose of 1% in artificial nectar while N-methyl-2-pyrrolidone (NMP), although less toxic to adults, was more acutely toxic to the brood. NMP is a member of a new generation of penetration enhancers added to pesticidal active ingredients to increase their systemic uptake into plants or animals. Use of novel microemulsion formulations of agrochemicals are often dependent on this and newer organosilicone adjuvants such as Silwet L-77. The latter at 0.1% significantly reduced learning performance of forager honey bees at 0.1% using the proboscis extension reflex (PER) assay.
Given the synergistic nature of certain chemicals, we are concerned that active ingredients may affect honey bees differently depending upon the inert ingredients in a particular product formulation or in certain combinations found in field use patterns. There is a new tendency to market pesticide blends including seed treatments that contain multiple classes of insecticides or fungicides, or their mixed combinations, that require these newer ‘inerts’ to achieve high efficacy. The fact that some inert ingredients may indeed be more toxic than active ingredients such as in formulations of the herbicide glyphosate makes this issue especially important for the health of honey bees and other pollinators.
Here this topic will be introduced with a broad overview of common inerts to be anticipated in pesticides frequently found in apiary samples. The constraints of analysis of these adjuvants in bee hive samples will be discussed. A short review of known impacts of surfactants and adjuvants on honey bees and other nontarget species will be given. We are in the process of identifying common inerts in pesticide formulations used frequently around honey bees or in their preferred foraging areas. The impact of synergistic pesticidal blends on bees, which depend on plant nectars and pollens readily contaminated by toxicants, cannot be fully understood without identification and risk assessment of inert residues and their agrochemical interactions.
21. Pernal, S.F., A.P. Melathopoulos, M.M. Guarna & L.J Foster – POTENTIAL FOR USING ANTENNAL PROTEINS TO SELECT FOR DISEASE AND VARROA RESISTANCE IN HONEY BEES
Though several resistance traits to American foulbrood disease (AFB) (Spivak & Gilliam, 1998 Bee World 79: 124-134, 169-186) and Varroa destructor (Rinderer et al., 2010 Apidologie 41: 409–424) have been identified previously, their inter-correlation and heritability in commercial breeding populations as well as their contribution to colony-level resistance remain poorly understood. We compared the distribution of AFB and Varroa resistance traits within two Western Canadian beekeeping populations assembled from queens derived from eight different regions: New Zealand, Chile, Hawaii, California, British Columbia, Alberta, Saskatchewan and Ontario.
At Beaverlodge, Alberta, half of the breeding population was tested for AFB resistance traits including: 1) hygienic behavior, 2) in vitro larval resistance to Paenibacillus larvae infection, 3) spore filtering efficiency of nurse bees, 4) in situ development of AFB after inoculation of larval patches, and 5) whole-colony development of AFB following inoculation with infected comb. In Grand Forks, British Columbia the remaining population, after being standardized for initial population size and density of V. destructor, was assayed for: 1) hygienic behavior, 2) Varroa sensitive hygiene (VSH), 3) phoretic and brood infestations levels of Varroa and 4) Varroa population growth over a ten week period. Quantitative genetic parameters were calculated from assay results for each trait between the parental and F1 generation, which was produced through a series of partial diallele crosses using instrumental insemination. Selection was performed on all phenotypic traits evaluated at Beaverlodge, and hygienic behavior and VSH for the Grand Forks population.
Results to date suggest that hygienic behavior and larval resistance to P. larvae each affect the expression of AFB in a colony environment but at different stages of the disease’s development. Preliminary estimates of heritability of traits between the parental and F1 generation of AFB-selected colonies indicate moderate heritability for hygienic behavior (h2=0.35) but no significant heritability for larval resistance traits. Estimates of heritability for hygienic behavior among Varroa-resistant-selected colonies indicate slightly lower heritability (h2=0.21). In addition, Varroa mite fertility was found to be negatively correlated with the expression of VSH and most colony measures of Varroa infestation were inter-correlated.
In parallel with the breeding and phenotype evaluation, biomarkers associated with disease or Varroa resistance were identified from adult and larval tissues using quantitative mass spectrometry-based proteomics. Comparative proteome profiling of honey bee antennal samples resulted in the relative quantitation of in excess of 1000 proteins. Using a linear mixed-effects model, proteins highly associated with hygienic behavior were discovered. Moreover, putative larval tissue and antennal protein biomarkers were identified that were significantly correlated with the expression of VSH as well as phoretic mite levels in colonies. These data show some promise that protein-based markers may hold future use in marker-assisted selection (MAS) programs for honey bees.
22. POLLARD, J., S. Cobeya & J. Chen – DEVELOPMENT OF PROTOCOL FOR THE INTERNATIONAL EXCHANGE OF HONEY BEE EMBRYOS
To enhance genetic diversity in domestic U.S. honey bee populations and to provide access to international breeding programs selecting for increased levels of resistance to pests and disease, there is a need to develop a standardized protocol for the safe and practical exchange of genetic stocks. Current techniques allow for the successful transport of honey bee semen which can be stored at room temperature with good viability for several weeks. Other constraints limit accessibility, semen collection requires specialized skill and equipment and the limited seasonal availability of drones in different climates can be difficult to coordinate.
Honey bee eggs are excellent candidates for stock exchange, provide a complete genetic package, are prolifically produced, and the three day egg stage allows for transport. We developed a method to transfer eggs in isolation, transport them a long distance, and rear queens from the transported eggs. The ability to collect and transport both semen and eggs would allow pure stocks to be established quickly.
Transport of honey bee germplasm reduces the risk of introducing pests and pathogens via live bees. Viruses are known to be carried in germplasm and remain a risk (Chen et al., 2006 App. Environ. Microbiol. 72: 606-611). To assess the relationship of viral transmission between donor queens and their eggs, commercial apiaries in California and Louisiana, representing the major U.S. commercial breeding populations, were surveyed. Queens, six months or older, and 50 of each queen’s eggs were collected from 100 colonies. Samples were analyzed using RT-PCR for the presence of eight viruses: acute bee paralysis virus (ABPV); black queen cell virus (BQCV); chronic bee paralysis virus (CBPV); deformed wing virus (DWV); Israeli acute paralysis virus (IAPV); Kashmir bee virus (KBV); sacbrood bee virus (SBV); and slow paralysis Virus (SPV).
Our results indicate that of the eight viruses tested, only three, BQCV, DWV and IAPV, were detected in both queens and eggs. The percentage of detectable virus was generally lower in the eggs as compared to the queens. This indicates transmission from the queen to her eggs may be reduced. DWV and BQCV were the most prevalent, although a higher rate of transmission between queens and eggs was observed with IAPV. Overall, these preliminary results suggest transmission of virus from the queen to her eggs does not appear to be strong and may vary among viruses.
The current semen importation procedure requires that the resultant colonies be established in quarantine for observation and testing. The delay in release of these colonies increases the risk of losing stock due to lack of forage and harsh winter conditions of the quarantine area. The establishment of a more efficient importation protocol for germplasm could allow for the direct release of stock, provided the risk of virus transmission is minimized.
23. Priscob, G.D., P. Francescob, C. Emiliob, F.H. Boncristiani, Jr., J.D. Evans & Y. Chen – VARROA DESTRUCTOR IS AN EFFECTIVE VECTOR OF ISRAELI ACUTE PARALYSIS VIRUS IN HONEY BEES
The alarming loss of honey bees, especially with Colony Collapse Disorder (CCD), poses a serious threat to the world’s food production, which relies heavily on honey bee pollination. The observation of the tight correlation between collapsed colonies and infection with the Israeli acute paralysis virus (IAPV) has put IAPV on the top of the list of suspects that may be responsible for the massive decimation and disappearance of honeybees. In the present work, we provide the first evidence that Varroa destructor, the most serious parasite of honey bees, is IAPV replication-competent and capable of vectoring IAPV in honey bees. In this study, honey bees were found to have become infected with IAPV after exposure to Varroa mites that carry the virus. The IAPV load in bees was positively correlated with the density of Varroa mites and time period of feeding by Varroa mites. However, bees originating from strong colonies tended to be more resistant to infection with IAPV, as shown by relatively lower levels of virus titer and higher levels of immune transcripts, compared to bees originating from weak colonies. This study has helped to define the role of Varroa mites in IAPV transmission and should shed considerable light on the epidemiology of IAPV infection in honey bees.
24. Rhoades, P.R., & J.A. Skinner – EFFECTS OF TREATMENT WITH THYMOL, FUMAGILLIN, HONEY-B-HEALTHY, AND NOZEVIT ON CAGED HONEY BEES INFECTED WITH NOSEMA APIS AND N. CERANAE
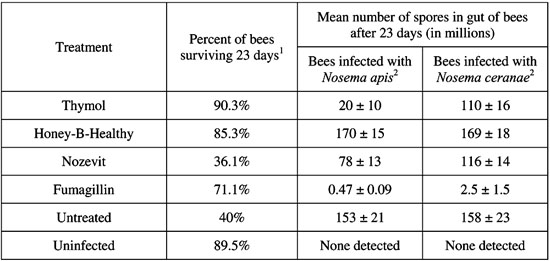
Prospective treatments for Nosema disease were evaluated by examining both mortality and spore production in caged honey bees infected with either Nosema apis or N. ceranae. Treatments used were thymol, fumagillin, Honey-B-Healthy, and Nozevit. Bees used in the study were shaken from frames of uncapped brood taken from hives visibly free of Nosema infection. These bees were chilled with ice water, placed in cages (180 bees/cage) and immediately infected with either N. apis or N. ceranae through gravity feeders at a rate of 40,000 spores/bee. Treatment began 3 days after inoculation. Dead bees were removed and counted every subsequent day. Twenty-three days after inoculation, 10-15 bees were removed, abdomens were crushed individually in water, and spores were counted using a hemocytometer.
Although treatment greatly affected mortality, no difference was noted with respect to the species of Nosema. Bees treated with thymol and Honey-B-Healthy were found to have rates of mortality similar to uninfected bees. Bees treated with Nozevit had rates of mortality similar to untreated bees. Fumagillin improved mortality, but not to levels of uninfected bees (Table).
Honey-B-Healthy had no effect on spore production; therefore, the reduction in mortality displayed under treatment must be attributed to positive effects on the bee itself rather than to an antagonistic effect on the pathogen. Fumagillin was the only compound to significantly and consistently reduce spore production. The failure of fumagillin to reduce mortality congruently is unexplained. Thymol and Nozevit had some effect on spore production but it was unclear how significant this effect was.
25. Rivera, R., F.A. Eischen, R.H. Graham and S. Piña – FATTY ACIDS IN HONEY BEE NUTRITION
Investigations have been done on honey bee nutrition focusing on the role of carbohydrates and protein. Research on honey bees’ need for dietary lipids is limited. The aim of this study was the determination of changes in fatty acids in the body of the honey bee with regards to nutrition. Lipids in the bodies of honey bees are essential structural components of cell membrane and cuticle. They provide a source of metabolic energy for sustained energy demand. Lipids in the body of honey bees include important hormones and pheromones, triglycerides, free and bound fatty acids, alcohols both long and short chain, mono-, di- and triacylglycerols, steroids and their esters, and phospholipids (Nation, 2002 Insect Physiology and Biochemistry, CRC Press). Honey bees synthesize and store lipids in the fat body tissue. These lipids and various amino acids are necessary for the synthesis of vitellogenin. Fat bodies are mostly found in the head and body cavity, especially in the abdomen.
Honey bees were collected from colonies fed pollen supplement, treated for Varroa and treated for Nosema in October, December and January. For fatty acid determinations, we ground up whole bees, extracted the fat, which was processed to fatty acid methyl esters for analysis by gas chromatography. Since fatty acids are the main fat reserves in honey bees, extraction of the whole body fat gives us an idea of composition of the fat in the fat body. A particular fatty acid, linoleic acid, has been reported to have antimicrobial activity against Bacillus larvae American foulbrood and Acosphaera apis chalkbrood (Feldlaufer et. al., 1993). We found eleven fatty acids that are of a quantitative concentration of importance. The major fatty acids determined on October bees were oleic acid 35%, linolenic 15%, palmitic 14%, and linoleic 10%, of total fatty acids present in unfed and untreated honey bees in October 2009. For colonies treated and fed pollen supplement, the relative amounts of these fatty acids changed by December, oleic acid 42%, linolenic acid 9.8%, palmitic acid 13%, linoleic acid 11%, and in January 2010 oleic acid was 35%, linolenic acid 18%, palmitic acid 13%.
A nutritionally important omega-3 unsaturated fatty acid is linolenic acid. Linolenic acid is a precursor to eicosenoid, which exerts control on reproductive biology and ion transport in insects (Stanley, 2006 Ann. Rev. Entomol. 51: 25-44). Linolenic acid increased in the bees that were fed pollen supplement. Omega-3and omega-6 fatty acids have important immunity and anti-inflammatory functions in human nutrition. In these honey bee samples omega-3/omega-6 ratio is 28.3, 22.9, and 32.3, respectively. October data is before treatment, December data, 1 month after treatment for Nosema, Varroa and fed shows impact on fatty acid concentration before rebounding in January.
26. Sammataro, D., J.A Yoder, J. Finely, R. Simonds, E. Olson & G. Degrandi -Hoffman – PESTICIDES IN POLLEN AND BEE BREAD
Thirteen specific fungi were identified in bee bread, including: Absidia sp., Alternaria sp., Bipolaris sp., Fusarium sp., Mucor sp., Penicillium sp., Rhizopus sp. and Trichoderma sp., Ascosphaera apis (chalkbrood disease), Aspergillus flavus (stonebrood disease), A. niger and Cladosporium. All were identified by culturing on potato dextrose agar and modified Melin-Norkrans agar.
Samples of bee bread were collected from colonies pollinating California almonds and from colonies in Arizona. Quantitatively, bee bread samples from California featured a reduced number of isolates of major fungal components (A. niger, A. flavus, Aspergillus spp., Penicillium spp., Cladosporium spp., Rhizopus spp.) than samples of bee bread from Arizona colonies. This is corroborated by results of our enumeration study where the total number of conidia in bee bread from California was nearly 3-5x less than number of conidia in bee bread from Arizona.
We tested the effects of fungicides on these bee bread fungi in the lab and found there was reduced growth and proliferation of fungi, which resulted in the production of fewer conidia, their primary mode of spread. Thus, fungal levels can be greatly reduced in bee bread. This may be a result of reduced amounts of conidia in a treated habitat; i.e., bees bringing pollen back to the colony that is coated with fewer conidia in a fungicide-sprayed habitat.
Our results show that A. apis was the fungus that was the most vulnerable of the bee colony fungi in that: 1) most of the bee colony fungi produced growth inhibitory substances against A. apis, suppressing spread and conidial output, thus there is overlapping sensitivity to chemical mediation by a wide range of fungi; 2) the presence of other fungi (A. niger and Rhizopus sp.) suppressed radial growth of A. apis by > 40%, which was the largest reduction in growth experienced by any of the fungi tested; and 3) a mycelium of A. apis was capable of being exploited and easily overgrown, with A. apis offering little resistance in the way of territorial defense once it establishes. The most versatile fungus was Trichoderma sp. in that the presence of this fungus had controlling effects on nearly all of the 13 fungi. If these fungi are missing, A. apis could increase.
27. Sheppard, W.S. & S.W. Cobey – IMPORTATION OF HONEY BEE GERMPLASM TO INCREASE GENETIC DIVERSITY IN DOMESTIC BREEDING STOCKS
The western honey bee, Apis mellifera, is the primary source of pollination for numerous U.S. agricultural crops, yet is an introduced species in the U.S. Most plants and animals of agricultural significance with overseas “origins” have benefitted from continued controlled importation of germplasm for breeding purposes. However, the honey bee remains an exception, as the importation of novel germplasm from the Old World for breeding purposes was severely curtailed with the passage of the 1922 U.S. Honey Bee Act. This Act and subsequent iterations restricted the importation of honey bee germplasm into the U.S. in an attempt to prevent the introduction of parasitic mites and, later, Africanized honey bees. Unfortunately, tracheal and Varroa mites became established in the U.S. in the 1980’s and by 1990, Africanized honey bees had expanded their range into the U.S.
Between 2008 and 2010, we collaborated with a number of U.S. and European queen producers and university laboratories on the collection of honey bee semen from three Old World honey bee subspecies (A. m. ligustica – Italy, A. m. carnica – Germany, A. m. caucasica – Georgia) for use in U.S. bee breeding programs. Under USDA-APHIS permit, we collected honey bee semen and then used it within one week to inseminate virgin queens provided by U.S. queen producers. Following virus-screening by USDA-ARS, these stocks have been released from WSU quarantine and provided to queen producers or maintained at WSU/UCD for further evaluation, incorporation into ongoing breeding efforts and release to the industry.
Recent progress in our laboratory resulted in the development of a functional cryopreservation methodology for honey bee semen, meaning that, for the first time, honey bee germplasm can be collected and stored for future use. In 2011, we will collect aliquots of semen from three honey bee subspecies of agricultural interest both for “standard” insemination (as was done previously) and also for long term liquid nitrogen storage. The overall goal of the collaborative project is to provide novel germplasm for honey bee breeding efforts, to collect and preserve Old World honey bee germplasm for future breeding efforts in the U.S. and to assist the U.S. bee breeding industry in its effort to develop superior honey bee stocks and to fully meet the needs of U.S. growers.
28. Smart, M. & M. Spivak – LOCATION, LOCATION, LOCATION: THE LANDSCAPE AND HONEY BEE HEALTH
The flow of protein from the external environment to the colony is integral to the survival and productivity of honey bees. Foragers collect pollen and bring it back to the hive where it is packed into cells and fermented through the assistance of beneficial microbes. Upon fermentation, the raw pollen is rendered more digestible for nurse bees that consume this “bee bread” and incorporate its protein into the development of their hypopharyngeal gland (HPG), a paired gland located in the head of the adult bee. Hypopharyngeal gland secretion, a major source of protein for developing worker brood, is then progressively fed to larvae as they develop inside their cells. Thus, the ability of bees to provide adequate nutrition to meet the demands of a growing colony is critically tied to the availability of pollen in the landscape. However, not all landscapes are equal and the overall quality of a particular environment for honey bees depends on many factors, including the diversity and abundance of floral resources, the nutritional quality of those resources, and the persistence of quality resources throughout the season. Various pollens differ in both their protein content and amino acid makeup, resulting in particular plants being more nutritionally complete for honey bees than others. Furthermore, diverse sources of pollen have been shown to have a positive effect on honey bee immune function when compared to single source pollens.
For these reasons we hypothesize that apiaries located in landscapes composed of a diverse and abundant range of forage (diverse sites) will be healthier and have greater survival throughout the season and into the following winter for pollination services than colonies located in areas primarily composed of non bee-friendly crops (poor sites).
Three diverse and three poor sites located in the Prairie Potholes Region of North Dakota were chosen based on historical honey yields, satellite imaging of crop coverage, and on-the-ground surveys of landscape floral composition. At each site, 24 colonies were sampled every six weeks for measures of colony strength, development, productivity, and stress levels (including pest, parasite, and pesticide levels). Furthermore, within each site, half of the colonies received additional protein supplementation while the other half do not, resulting in colonies within each landscape (diverse or poor) that were with and without an additional protein source.
Individuals were sampled to determine their nutritional status (HPG size and protein content, fat body lipid levels) and immune function (expression of anti-microbial peptide gene transcripts). Currently data is only available on HPG protein. Hypopharyngeal glands were larger (p<0.0001) in bees residing in diverse landscapes than those at poor sites. Additionally, diverse site bees with and without protein supplementation had significantly larger glands than bees at poor sites both with (p<0.05) and without (p<0.05) protein supplementation. Hypopharyngeal gland protein content was greater in diverse site bees, though not significantly. However, diverse site and poor site colonies that received protein supplementation had greater HPG protein content (p<0.05) than bees at poor sites with no protein supplementation. Gland size and protein content had a significant positive correlation (Pearson=0.6228, p<0.0001) indicating that bees with larger glands also had higher levels of protein in their glands.
Future work includes continued analysis of protein, lipid, and immune function to further discern physiological differences of honey bees in varying landscapes. Additionally, study of the biological and colony relevance of varying HPG and size and protein content, and other physiological measures will be further pursued and studied.
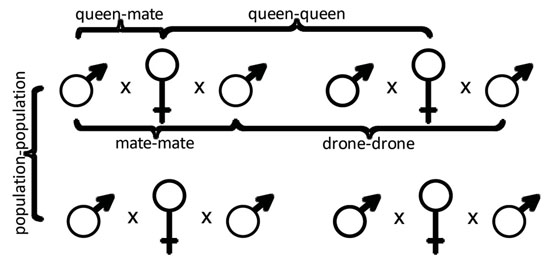
29. Tarpy, D. R., J.R. Caren & D.A. Delaney – META-ANALYSIS OF THE GENETIC RELATEDNESS AMONG HONEY BEE DRONES, QUEENS, AND THEIR MATES
Honey bee colonies are large kin groups, each with a single mother queen and thousands of female workers. Queen bees are highly polyandrous, each mating with an average of approximately 12 drones from other colonies. We used a meta-analysis approach to compare the pedigree relationships of honey bee reproductives (queens and their mates) across five different studies and to quantify the overall genetic diversity of breeding populations. We compared the inferred genotypes of queens and their mates from microsatellite analyses of worker offspring from a feral Africanized honey bee population (which served as a negative control for inbreeding), an experimentally derived population of sister queens (which served as a positive control for inbreeding), and three separate commercially managed populations. We then compared the relatedness of all drones mated to each queen (mate-mate), all queens within each population (queen-queen), each queen with each of her mates (queen-mate), and all drones within each population (drone-drone) (Figure). We found, as expected, the lowest levels of genetic similarity in the outcrossed population and highest levels of genetic similarity in the inbred population. Levels of genetic similarity among the managed honey bee populations were intermediate but closer to that of the inbred population. The effects that these findings have on the overall population genetic diversity of managed honey bees will be discussed.
30. Teeters, B.S., R.M. Johnson, B.D. Siegfried & M.D. Ellis – BEES UNDER SURVEILLANCE: USING VIDEO-TRACKING TO MONITOR HONEY BEE ACTIVITY AFTER SUBLETHAL EXPOSURE TO PESTICIDES
Abiotic stress from the sublethal effects of pesticides is currently being scrutinized as a contributing factor to poorly understood honey bee colony losses. The current approach to risk assessment does not require data on sublethal effects prior to product registration. However, recent studies have found that sublethal doses of some pesticides can compromise honey bees’ immune system and also synergistically interact with other pesticides used in beekeeping and on orchard crops. The recent trend in crop pest management practices toward the use of systemic pesticides is also a potential source of abiotic stress. Systemic toxins are translocated to all plant tissues, including nectar and pollen. All the above factors support the need to establish effective assessments of sublethal effects in the risk assessment process.
For this study, a video-tracking software system, EthoVision® XT, was employed to monitor the activity of honey bees exposed to sublethal levels of tau-fluvalinate and imidacloprid. Tau-fluvalinate is used in the hive for Varroa mite control while imidacloprid is a commonly used systemic neonicotinoid and has been shown to affect bees after exposure to concentrations as low as 24µg/kg (Decourtye et al, Ecotoxicol. Environ. Saf. 57: 410-419). Worker bees were treated topically with 1µl of tau-fluvalinate diluted to 0, 0.3, 1.5, or 3µg/ml acetone. The imidacloprid was administered orally in a sugar-agar food containing 0, 0.005, 0.05, and 0.5ppm imidacloprid. Each treatment used 4 pairs of workers and was replicated 4 times per trial.
EthoVision® XT measured differences between treatments over 24 hours in the distance that individuals moved and the amount of time spent near the sucrose, as well as the amount of time that a pair of bees spent interacting. The observations were repeated 4 times with different bees. For distance moved, bees treated with the middle and highest doses of tau-fluvalinate moved significantly less than control bees (p-value 0.004 and 0.001, 30m vs. 50m) as did bees exposed to 0.05 ppm imidacloprid (p-value 0.025, <20m vs. 35m). The difference between control and 0.005 ppm imidacloprid was approaching significance (p-value=0.0519), suggesting a possible stimulatory effect at this level. Bees treated with 1µl of 0.3µg/ml tau-fluvalinate spent more time interacting (p-value 0.001) than controls, with about a 2-fold difference, while bees exposed to 0.05 and 0.5 ppm imidacloprid spent less time together than controls (p-value 0.0002 and 0.002). There was no significant difference between the control and any dose of tau-fluvalinate in the amount of time spent in the food zone, but bees exposed to 0.5ppm imidacloprid spent significantly more time there (p-value 0.003, 7hrs vs. <1hr).
EthoVision®XT made it possible to quantify sublethal effects on bee activity, and such systems may provide a novel means of assessing the risks associated with sublethal exposure to toxins in honey bees and other pollinators. However, it is necessary to correlate laboratory results to field conditions to establish whether the changes in activity documented in these assays have a significant effect on colony performance. EthoVision®XT results must be related to colony performance to make the procedure useful in risk assessment.
31. Traver, B.E. & R.D. Fell – NOSEMA CERANAE INFECTIONS IN DRONES OF DIFFERENT AGES
Nosema ceranae is a widespread microsporidian pathogen of Apis mellifera and is found on all continents where beekeeping is practiced. Recent studies suggest that N. ceranae has been displacing the other microsporidian pathogen of honey bees, Nosema apis (Klee et al., 2007 J. Invertebr. Path. 96: 1-10). Nosema apis transmission is thought to be primarily through a fecal-oral route that may occur during cleaning of contaminated comb, consumption of contaminated food and/or water, and through trophallaxis. Transmission of N. ceranae may have similar mechanisms as N. apis, but due to what appears as a relatively recent and quick displacement, mechanisms other than a fecal-oral route may be involved in the transmission of N. ceranae. The objective of this research was to determine whether drones could be involved in the transmission of N. ceranae. An estimated 40-60% of drones drift from their parent colony and of those, 21% drift more than once (Currie & Jay, 1991 J. Apic. Res. 30: 61-68). Drones could thus provide a mechanism for transmission of N. ceranae both within and between apiaries.
In this study, drone pupae (purple eye stage, body pigmentation, approximately 17-23 days of age), drones found in the hive, and drones returning from orientation or mating flights were collected and analyzed for Nosema spp. Both quantitative real-time PCR (qPCR) and spore analysis were used for diagnosis. Duplex qPCR was performed on pools of drones to determine infection by N. ceranae and/or N. apis and at what level. Spore counting was also performed on pools of drones to estimate the average number of spores per drone. In all of the colonies, workers were sampled to confirm that the colony was infected with N. ceranae and to estimate the level of infection. All colonies were positive for N. ceranae and negative for N. apis.
Preliminary analysis showed that drones of all ages were infected at low levels. Our results indicated that infection in drones were most prevalent from April through June. As a trend, drone pupae were more frequently found to be infected in June. In-hive adults were most highly infected in May and June. Flying drones were only sampled in July and August, and tended not to be infected, only 5.1% (2/39) samples showed a moderate to high level of infection. These findings indicate that drones could assist in the transmission of N. ceranae between hives within an apiary.
32. Tsuruda, J.M., J.W. Harris, L. Bourgeois, R.G. Danka & G.J. Hunt – USING SINGLE-NUCLEOTIDE POLYMORPHISMS AND GENETIC MAPPING TO FIND CANDIDATE GENES THAT INFLUENCE VARROA-SPECIFIC HYGIENE
Varroa-sensitive hygienic (VSH) behavior is one of two behaviors identified that are most important for controlling the growth of Varroa mite populations in bee hives. A study was conducted to map quantitative trait loci (QTL) that influence VSH so that resistance genes could be identified. Crosses between high- and low-VSH lines resulted in a backcross family. Individual workers were tagged with numbered disks and evaluated for their VSH behavior in a mite-infested observation hive. Bees that uncapped or removed mite-infested pupae were identified. Probes for 1,536 single-nucleotide polymorphisms (SNPs) within genes were used to analyze the genomic DNA of worker bees in this backcross family. The genotypes for 1,348 informative SNPs were used to construct a high-resolution genetic map using JoinMap software and to compare genotypes of individuals that performed VSH behavior to those that did not perform the behavior. Interval mapping using MapQTL software identified one major QTL on chromosome 9 (LOD score=3.17) and a suggestive QTL on chromosome 3(LOD=2.16). The QTL region on chromosome 9 contains relatively few genes and the center of this region contains a dopamine receptor. This type of dopamine receptor has been previously shown to be required for olfactory and aversive learning, which are necessary for identifying mites within brood cells.
33. Webster, T.C. & M.A. Matisoff – PRIMARY SPORES OF NOSEMA CERANAE ARE EARLY INDICATORS OF INFECTION
The early stages of Nosema ceranae development include the formation of primary spores. These are visibly different from the environmental spores which develop later. Hence, observations of primary spores may allow relatively quick evaluations of treatments for Nosema disease and evaluations of Nosema-resistant stocks of honey bees. Both N. ceranae and N. apis generate primary spores within four days of inoculation. Nosema apis and N. ceranae infect the adult honey bee through a complex process that includes two types of spores [Fries et al., 1992 Apidologie 23: 61-70; Solter, 2010 Amer. Bee J. 150(12) and Bee Culture 138(12)], and this is common to many Microsporidia (Cali & Takvorian, 1999 in The Microsporidia and Microsporidosis. M. Wittner and L. M. Weiss, eds). Environmental spores are those that survive outside of the bee. When ingested, these spores germinate within the midgut and evert a polar filament or tube. This very long tube will pierce the bee’s midgut epithelial cells, and the sporoplasm then moves from within the spore, through the tube, and into an epithelial cell. There the sporoplasm reproduces to generate primary, or autoinfective, spores. These primary spores then invade adjacent midgut epithelial cells, multiply, and finally produce the environmental spores. When the epithelial cell ruptures, environmental cells are released into the midgut lumen. Ultimately, these spores are released from the bee in fecal material.
Caged bees fed N. ceranae spores in sucrose syrup were sampled 4, 6 and 12 days afterward. The mean primary spore loads were 10,000, 105,000 and 817,000 spores per bee, on those dates respectively. Caged bees fed N. apis spores and sampled at the same dates developed mean primary spore loads of 3,000, 34,000 and 431,000 spores per bee, respectively.
34. Wilson, M.B., A.D. Hegeman, M. Spivak & J.D. Cohen – DETERMINING THE BOTANICAL ORIGINS OF PLANT RESINS COLLECTED BY APIS MELLIFERA WITH METABOLIC FINGERPRINTING ANALYSIS
Honey bees (Apis mellifera) collect resins from plants in their environment and deposit them in their nests. Resin production in plants is a defense mechanism that prevents both infection and predation, and honey bees utilize resins in much the same way. In temperate regions of Europe and the United States, honey bees collect resin primarily from the young leaves and buds of trees in the Populus genus (poplar, cottonwood, and aspen) (Simone-Finstrom & Spivak, 2010 Apidologie 41: 295-311). ‘Propolis’ (pro – “at the entrance to”, polis – “city”) is the apicultural term used to describe plant resins once they have been mixed with wax and deposited as a coating or glue in the nesting cavity.
The antimicrobial properties of propolis have been known since antiquity (Ghisalberti, 1979 Bee World 60: 59-84) and propolis in the hive decreases immune-related gene expression in adult bees, possibly by decreasing the abundance of bacteria in the hive (Simone-Finstrom, 2009 Evolution 63(11): 3016-3022). Chronically activated immune expression decreases productivity in honey bees (Evans & Pettis, 2005 Evolution 59(10): 2270-2274), so it is important that bees externally supplement their innate immune function with antimicrobial resins. The mechanism(s) by which this occurs is not well established, although the botanical origin and chemical composition of resins must play a role. We want to identify the botanical sources of propolis, which will allow us to ask questions about the relationships between bees and resin metabolites in the environment.
Previous studies have used observational or limited metabolite analysis to determine the botanical sources of propolis (Park et al., 2002 J. Agri. Food Chem. 50: 2502-2506; Santos et al., 2003 Phytother. Res. 17: 285–289). Direct observation of resin-foraging is difficult because resin-foraging is a relatively rare event and may occur high above ground level (Crane, 1990 Bees and Beekeeping: Science, Practice and World Resources, Heinemann, London, UK). Metabolite analysis is a good alternative; however limited analyses may paint an incomplete picture because resins are chemically complex. We have taken a metabolomics approach to this problem by measuring as much of the entire population of metabolites in propolis as possible with chromatographic/spectral “fingerprints” that can be used to determine the botanical origins of propolis without extensive prior knowledge of bee resin-foraging preferences or biochemistry of foraged plants.
We used multivariate statistical tools (principle component analysis and partial least squares discriminate analysis) to (1) classify fingerprints of propolis samples from botanically discrete regions based on metabolite patterns and (2) identify specific metabolites that were either reproducibly similar or different among fingerprints. Metabolic fingerprints of resins collected from resin-foragers returning to the hive (before resin is deposited in the nest) and resins from local woody plants at University of Minnesota St. Paul campus apiaries indicate that propolis in our area is derived from a Populus genus hybrid.
It may be that certain resin producing plant species are more beneficial to honey bees than others. With methods to discover both the botanical sources of propolis as well as specific metabolites important its biological function, we may ultimately be able to link the immunological benefits of propolis to bees’ botanical environment.
35. Zhu, W., D. Schmehl & J. Frazier – MEASURING AND PREDICTING HONEY BEE LARVAL SURVIVAL AFTER CHRONIC PESTICIDE EXPOSURE
For years, the agricultural communities are working under the assumption that the pesticides are safe for bees if applied according to the manufacturer’s label on the lethal values of pesticides based on adult bees. However, there is compelling evidence that the assumption is simply not true. We used a modified in vitro larval rearing technique to assess the survival of honey bee larvae under 6-day chronic exposure to sublethal doses of four pesticides (Fluvalinate, Coumaphos, Chlorothanil, and Chlorpyrifos), which are the most frequently found pesticides in bee pollen. Based on the proposed Probabilistic Toxicity Model, we demonstrated the significant difference in the sensitivity to pesticides between honey bee larvae and adult bees. The observed larval mortality caused by Chlorothanil, which is a common fungicide allowed to be freely applied during flower blooming, can be 6 times more than the predicted adult mortality. The algorithm of relative toxicity was proposed to compare the toxicity of each pesticide for honey bee larvae under the same-scaled toxic index. The relative toxicity of Chlorothanil is almost 40 times higher than that of Chlorpyrifos. Moreover, considering the pesticide formulation, we demonstrated the high toxicity of N-Methylpyrrolidone (NMP) to honey bee larvae. A 1% dose of NMP can kill all the reared larvae during the first day.
Another concern for pesticides is complex interaction among different pesticides. It was shown that Fluvalinate and Chlorothanil have significant synergistic interaction at higher concentration, however, at a lower concentration, this interaction can be antagonistic. Our study suggests that fungicide, the inert ingredient and pesticide interaction should be of high concern to honey bee larvae and overall colony health. None of these factors can be neglected in the pesticide risk assessment for honey bees.
Video Recordings
- ABRC2011 The Good the Bad and the Ugly Varroa Mite Control Strategy
- ABRC2011 The CAP Stationary Apiary Project: A Synopsis of Colony Strength and Preliminary Statistical Modeling 2009-20010
- ABRC 2011 Varroacide Effects on Honey Bee Queens
- ABRC 2011 Bees Under Surveillance: Using Video-Tracking to Monitor Honey Bee Activity After Sublethal Exposure to Pesticides
- ABRC 2011 Evaluation of Honey Production and Varroa Mite Load in Russian and VSH Bees
- ABRC 2011 Broodmapper: Developing a Web-Based Game to Detect Pesticide Effects in Honey Bee Brood
- ABRC 2011 Report on Pesticides Found in Pollen and Bee Bread in Colonies Placed in California Almond Orchards
- ABRC2011 A Primer on Pesticide Formulation ‘inerts’ and Honey Bees
- ABRC2011 Challenges for Developing Biopesticides Against Varroa
- ABRC 2011 Relay Effect in Landscape Learning in Honey Bees and its use for Detecting Subtle Effects of Pesticides.
- ABRC 2011 Examination on the Reproductive Success of Re-invading Varroa Mites
- ABRC 2011 Determining the Botanical Origins of Plant Resins Collected by Apis mellifera with Metabolic Fingerprinting Analysis
- ABRC 2011 Location Location Location How Landscape Quality Affects Honey Bee Health
- ABRC 2011 Bee Communities at Crop Flowers and Bee Food Plots
- ABRC 2011 Identifying Candidate Genes for Honey Bee Mite-Grooming Behavior Using Fine-Scale Linkage Mapping