A National Research and Extension Initiative to Reverse Pollinator Decline
This is part of an ongoing series of updates from the Managed Pollinator CAP. Additional installments can be found at the:
More information about the CAP can be found at:
CAP Updates: 6
Tom Webster, Kentucky State University, Frankfort, KY
- Jointly published in the American Bee Journal and in Bee Culture, April 2010.
Quite a bit has been written on Nosema ceranae in recent years. This pathogen was discovered in European-stock honey bees only a few years ago. Now we know it to be a worldwide disease. Much of our understanding of N. ceranae is based on a century of research on a related disease, Nosema apis. N. apis is the “nosema disease” discussed in many honey bee books. It often weakened colonies without killing them. But this newly discovered N. ceranae is more serious, and the biology is different in significant ways. Collectively, they are called “Nosema” in this article.
This article will cover some of the fundamental issues and focus on what I feel are the most important findings. We can organize our understanding and long range plans into the areas of biology, diagnosis and control. Biology comes first, because it is essential to good diagnosis and effective control. Careful diagnosis is essential to beekeeping, and a little tricky because the symptoms of Nosema can be ambiguous. Treatments beyond fumagillin, sold by the trade name Fumagilin, have been hard to come by. This microbe is well protected either by the spore wall, or by the honey bee tissue it invades.
Biology
Much of our understanding of both Nosema species is illuminated by a vast amount of work on the larger group called the Microsporidia. This is a group of parasitic fungi, and it includes over 200 different species of Nosema. These pathogens have much in common, and some even infect humans. Later this year, Dr. Lee Solter will contribute an article describing the big picture of these pathogens and their hosts, and how they inform us of honey bee pathology.
Like other Microsporidia, the Nosema organism exists as a spore form and a vegetative form. The spore is a single cell with a tough coat around it. Like spores of American foulbrood or chalkbrood, it can survive for years on beekeeping equipment. We must direct our control efforts at both forms. But the strategy for spore destruction is very different from the efforts to kill the vegetative form. An infected bee usually has both spores and vegetative forms.
The infection begins when a bee, usually a worker, consumes spores. This can happen when a house-cleaning bee removes feces from comb, and ingests some spores from the feces. The spores then travel through the esophagus, the crop, and then into the midgut. The midgut is where the bee produces enzymes to digest pollen and honey, and absorbs the nutrients in those foods. Some sort of stimulus then causes the spores to germinate. This means the spores each shoot out a very long, thin tube called the polar filament. See Fig 1. This filament is so long and moves so quickly, that it often reaches one of the cells that line the inside of the bee’s midgut.
After the filament penetrates the bee’s midgut cell, the remaining contents of the spore migrate through the filament. This is the infective machinery of the Nosema organism, the “sporoplasm”. When the sporoplasm enters the bee’s cell, trouble begins. The Nosema hijacks the bee’s cell processes, and begins to grow and multiply. This is the vegetative form of the disease. Soon the bee’s cell is entirely dominated by the developing Nosema, and new spores form. Some spores invade adjacent bee midgut cells. Others are shed when the midgut cell breaks open. These new spores may germinate in the midgut and infect more cells. Or they may pass on to the rectum of the bee, and come out in feces.
Several new twists on the story have come to light with recent research. N. ceranae DNA has been found in the hypopharyngeal glands of infected worker bees, and in stored pollen in infected hives. The hypopharyngeal glands secrete much of the food for bee larvae and the queen. Perhaps this is another mode of disease transmission.
Diagnosis
It is quite common for beekeepers to treat their bees for Nosema without knowing whether they have the disease. This can be an unnecessary expense, and possibly harmful to the bees. However, diagnosis is not always easy. The following are methods for diagnosis, each with its advantages and problems.
Spotting on the front of the hive. Bees with problems in their digestive system will often defecate on the front of the hive, as they exit and crawl up from the entrance. See Fig 2. This “spotting” can be caused by Nosema infection, and apparently by other disorders. So this symptom suggests Nosema but is not a sure indicator of disease. On occasion I have collected this fecal material from the front of a hive and found no spores when I examined it by microscope.
The “field test”. One popular test is to pull the midgut from a bee and examine it for discoloration. Often, a healthy midgut will appear reddish brown, while a bee with Nosema will have a white or cream-colored midgut that is swollen. A beekeeper can examine a bee in the field, by pulling the gut out with tweezers (Fig. 3). A magnifying glass is helpful.
But this field test is also unreliable. See the midguts in Fig. 4 . The midguts numbered 1 – 10 were taken from healthy bees I collected at the hive entrance on a nice day. Those numbered 11 – 20 were heavily infected, after consuming spores in laboratory cages. The two sets of midguts look about the same. So appearance cannot be a good indicator. I suspect that a white or creamy, smooth midgut can be caused by other microbes, perhaps as secondary infections that may or may not occur with Nosema. Also, pollen in the midgut adds to the color and varies widely according to the floral source of the pollen.
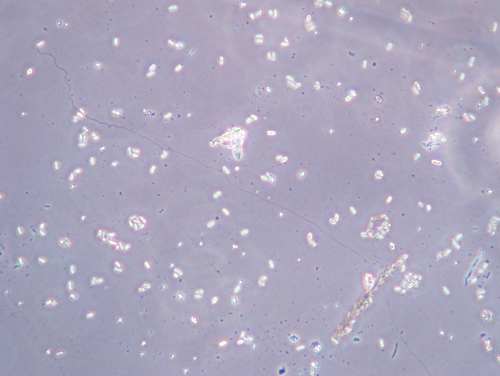
Examination of gut contents with a microscope. For a rapid and accurate diagnosis, a good microscope is the tool of choice. A sample is prepared by squashing the guts of bees in water, and placing a drop of the liquid onto a microscope slide. A magnification of 400 power is best. The spores are seen as ovals, about 3 microns by 5 microns. N. apis spores tend to look like “racetrack” ovals : flat on the sides and round at the ends. N. ceranae spores are more almond shaped, and slightly smaller. However, there is wide variance in shape among the spores, so we cannot rely entirely on what we see. Spore shape suggests the species of Nosema, but is not conclusive evidence. The vegetative form of the disease is there too, but difficult to see with a standard microscope. A hemacytometer is a special type of microscope slide that allows one to count the number of spores in a small volume of water, and estimate the total number of spores per bee. A heavily infested bee may have over 20 million spores of either type of Nosema.
The antibody test. This test was described by Dr. Kate Aronstein in the January 2010 issue of this journal. It has very important promise because it will be rapid, simple, and will not require the expense of a microscope.
Genetic methods. Tests for DNA specific to a species of Nosema is the gold standard. A lab method called polymerase chain reaction allows the identification of minute amounts of DNA from either N. apis or N. ceranae. However, this test is laborious and requires expensive equipment and considerable expertise.
Other possibilities. I have seen extensive fecal debris on the inside of a N. ceranae-infected hive. Feces were on the top bars and underside of the inner cover. My observations were in July, when the bees could fly nearly every day. This hive appeared healthy in other ways. This raises the possibility that spore transmission via fecal debris can happen in good weather, not just when the bees are confined indoors during a long winter.
Controlling Nosema disease
Control of vegetative forms inside the bees.
Fumagillin. This chemical has been used for many years to control Nosema in honey bees and related pathogens in other animals and in humans. It is often still effective against both Nosema apis and Nosema ceranae. However, we must consider alternatives. We cannot rely on a single type of treatment. Experienced beekeepers have seen the development of varroa mites highly resistant to chemical controls and American foulbrood bacteria resistant to antibiotics. The same problem will certainly arise with extensive use of fumagillin.
Fumagillin is produced naturally by another type of fungus called Aspergillus. Like many microbes, Aspergillus has developed its own arsenal of chemicals that help it survive.
Fumagillin treatment is prepared in sugar syrup, and fed to bees. Inside the midgut, it penetrates Nosema-infected cells. There it interferes with the Nosema genetic machinery, or with certain proteins. It does not affect the spores. After fumagillin treatment, the midgut will still contain viable spores unless the infection is in its earliest stage, before spores have formed.
Beekeepers should be aware that Nosema spores will persist inside the bees long after the fumagillin treatment. Even if all of the vegetative forms are killed by fumagillin, spores will persist, possibly for weeks. Some beekeepers who collected bees from their hives a day or two after treatment have been alarmed to see that the spore counts did not go down. From this, they incorrectly assumed that the treatments were ineffective. Beekeepers should wait for at least several weeks after the bees consume the fumagillin before sampling again for Nosema.
Beekeepers should also be aware that fumagillin will degrade quickly in light, especially sunlight. It should not be fed to bees in clear, glass jars that are exposed to sunlight. Also, it is heat sensitive. Fumagillin should be added to syrup after it has cooled. Many other products have activity against the vegetative form of Nosema. Unfortunately, most are either less effective than fumagillin or more expensive. The difficulty is that a product must enter the honey bee midgut cells to kill the Nosema. And it must not seriously affect the very sophisticated and complex honey bee colony behavior and physiology. However, I am somewhat optimistic that effective and relatively inexpensive treatments will be discovered. We can consider that plants and animals have been battling fungal diseases for over a billion years. That has given them plenty of time to develop their own natural defenses. One such product is now under study here at KSU.
Control of spores outside of the bees, by comb and equipment decontamination.
Now that N. ceranae is known to be widespread in the U.S. and other countries, many beekeepers must consider methods for decontamination of equipment. If a bee colony dies or is weakened by the disease, we must assume that much of it is contaminated with spores. Healthy bees on this equipment will quickly consume spores by their comb-cleaning behavior, causing the disease cycle to resume. Destruction of the equipment would be effective, but expensive.
Several agents do kill spores. One is ultraviolet (UV) light. In Fig 5 we see spores killed by UV light. The killed spores are bright red with a fluorescent stain, which enters spores with damaged membranes. Years ago, in a more civilized time, people dried their laundry outdoors on something called a clothes line. This often sterilized the clothing as it dried, because sunlight is a potent source of UV light. UV light kills Nosema spores quite effectively and it leaves no chemical residue. The main problem is that beekeeping equipment must be manipulated quite a bit to allow light, from the sun or a UV lamp, to get exposure into all the cells and wooden parts. This may be an option for some small scale beekeepers.
A variety of liquids, including dilute bleach and alcohols, also kill spores. However, these methods also require considerable equipment manipulation. The equipment must be dried and free of fumes before it could be used by the bees.
Thomas C. Webster
Land Grant Program
Kentucky State University
Frankfort, KY 40601