Introduction
European foulbrood (abbreviated EFB) is a bacterial disease that effects honey bee larvae before the capped stage. European foulbrood disease is characterized by dead and dying larvae which can appear curled upwards, brown or yellow, melted, and/or dried out and rubbery. The causative bacteria, Melissococcus plutonius is ingested by honey bee larvae after which the bacterium competes for food inside the larvae. If the bacteria out-competes the larva, the larva will die before the cell is capped. Alternatively, the bee may survive until adulthood if the larvae has sufficient food resources. European foulbrood should not be confused with American foulbrood (AFB), which is caused by a different bacteria that produces different symptoms and control requirements.
European foulbrood disease is considered to be more problematic in situations where forage nectar is sporadic, or other situations that result in fewer nurse bees in colonies to feed larvae. At the onset of nectar flow in early spring, forage recruitment of house bees may increase rapidly resulting in few bees in colonies to feed honey bee larvae. Often, when the nurse bee to larvae ratio stabilizes later in the season, or remains stable throughout a season, symptoms disappear. However, this disease can occur throughout a season and will sometimes not clear up on its own. In severe cases, colony death can occur. Also, yearly reoccurrence of EFB from contaminated combs and equipment can occur. The bacteria that causes EFB does not produce spores, but combs contaminated with the bacteria can still reinfect honey bees in subsequent years.
Causative agent
European foulbrood is caused by the bacterium Melissococcus plutonius. G. F. White is credited with first identifying the correct bacterium that causes European foulbrood in 1908, naming it Bacillus Y which he later renamed Bacillus pluton (Baily 1983). The bacterium was subsequently renamed by several scientists after it became clearly linked to the disease. Baily (1956) isolated the bacterium and, based on morphology, called it Streptococcus pluton. Baily and Collins (1981), later re-classified the bacterium as Melissococcus pluton based on additional culture and chemical knowledge. This was then tweaked due to nomenclature rules to Melissococcus plutonius meaning “pertaining to Pluto or the underworld” instead of M. pluton which means “Pluton, Greek god of the underworld'” (Truper and de Clari 1998).
As if the changing nomenclature is not enough to confuse, several other bacteria that are only found in association with M. plutonius were at times credited with causing EFB. This is at least partially due to the fact that these bacteria can overgrow M. plutonius and sometimes seem to improve its growth in lab conditions (Baily 1983). These secondary, infective bacteria present with M. plutonius include; Paenibacillus alvei, Achromobacter (Bacterium) eurydice, and Bacillus laterosporus Laubach (Shimanuki 1997). These bacteria are sometimes considered symbiotic and may cause some of the differences in smell and appearance in infected larvae (Baily 1981). There is suspicion that some of these bacteria may have some causal relationship to symptom onset, but this has never been clearly established (Shimanuki 1997).
Life cycle of European foulbrood
Larvae become infected with European foulbrood when they consume brood food that contains the bacteria M. plutonius (Shimanuki 1997). There is also some evidence that transmission may occur from bites of the parasitic mite, Varroa destructor (Kanbar and Engels 2003). Inside infected larva, the bacterial populations concentrate in the food mass in the midgut and the gut peritrophic membrane interface (McKee et al. 2004) where the bacteria then reproduces (Bailey 1983). Depending on the level of infection, and possibly the amount of available food, the infected larva will either survive or die.
The degree of larval mortality, measured in one experiment, was directly related to the duration or amount of bacteria that was fed to the larva. Larvae were found to be more likely to die as increasing amounts of bacteria were fed (McKee et al. 2004). The larvae that survive go on to defecate and pupate, which leaves bacteria on the combs that can be infective for years, even though this bacteria does not produce spores (Baily 1981, 1983). Surviving larvae will become adults with generally lower weight and delayed pupation when compared to their uninfected counterparts, supporting the idea that infection creates higher energy demands (Baily 1960, Mckee et al. 2004). It is noted that an increased food supply from adequate numbers of nurse bees can reduce larval death and observed symptoms. With adequate amounts of food, larvae are more likely to survive (Baily 1983). This may explain why expression of the disease can change sporadically year to year, and season to season, depending on the balance of nurse bee to larvae ratio and thus, the amount of brood food made available to the larvae.
Symptoms of European foulbrood
It is important to not confuse European foulbrood with American foulbrood. These are two very different diseases that require different management and treatment routines. Both are however bacterial brood diseases. Use the table below as an overview to tell the difference between European foulbrood and American foulbrood.
| European foulbrood | American foulbrood |
|
|
|
Fig.3: Table from Shimanuki and Knox (2000) and Delaplane (1998), Ropey length from Shimanuki (1997), American foulbrood photo by Williams, USDA. |
|
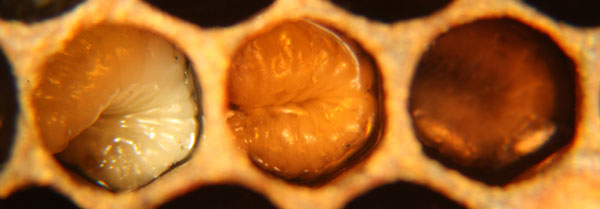
A “spotty brood pattern” (Fig. 4) in a honey bee colony can often be the first sign of a wide variety of problems, including EFB. A spotty brood pattern can occur when some larvae die in their cells from a disease, while others survive and become capped resulting in a spotty or shotgun appearance of the capped stage of brood. Many other conditions and situations can cause a spotty brood pattern. For example, an inbred queen can produce a spotty brood pattern when the alleles at the sex locus become homozygous. This produces fertilized, diploid males which are then consumed by worker bees. Although not unique to hives affected with EFB, a spotty brood pattern is a common symptom of EFB.
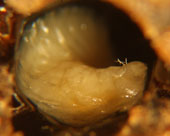
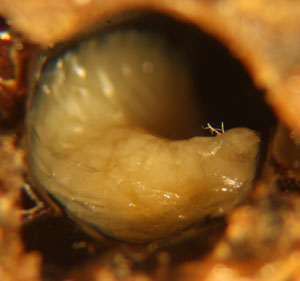
In hives infected with EFB, dying and dead larvae can become yellow and then brown. A sour, fishy odor may be present or not. Tracheal tubes can become more apparent as the larvae flattens or ‘deflates'(Fig.2). The larvae can also twist as they die and can die curled upwards (fig.1). Other times they melt in their cells and will generally be mushy. The remains can be slightly ropey with threads less then 1.5cm long (Shimanuki 1997). To test if the remains are ropey, a toothpick, match, or small stick can be probed into the cell and removed (fig.5). Once dried, a rubbery scale remains.
Confirming diagnosis
Diagnosis of infection with European foulbrood should begin with visual inspections of the above symptoms. Beekeeper’s inexperienced with EFB would likely benefit from confirmation of diagnosis before taking action, in case the infection is another bacteria, virus, chilled brood, or some other situation. Confirmation could occur through their state sponsored apiary inspection program, if available, or by the use of an inexpensive and easy to use diagnostic test kit, or sending a sample to the USDA Beltsville Bee Research Laboratory for testing.
Diagnostic field validation kits for EFB are based on monoclonal antibodies of M. plutonius (Tomkies et al. 2009). Samples sent to the USDA Beltsville Bee Research Laboratory for diagnosis will be examined microscopically for the presence of M. plutonius or one of the associated bacteria, which have often eliminated M. plutonius (Smith 2009). Other methods to confirm M. plutonius include: Enzyme-linked immunosorbent assays (ELISA), (Pinnock and Featherstone 1983), a hemi-nested PCR assay (Djordjevic et al. 1998), and quantitative real-time PCR (Roetschi et al. 2008).
For additional information on diagnosing European Foulbrood, see the following two pages.
Occurrence and distribution
European foulbrood occurs on all continents where honey bees are kept (Shimanuki 1997). During the early 1980’s in the U.S., it was historically severely problematic in New Jersey during the spring cranberry and blueberry pollination season. This created some suspicion that low nutrition in pollination fields were having an effect on EFB occurrence, but this did not seem apparent in trials conducted by the USDA (Herbert and Shimanuki 1984). This regional outbreak in New Jersey pollination fields was not consistent. Herbert et al. (1987) reported that in 1986-87, European foulbrood could not be found in the New Jersey, USDA test colonies, while previous to 1986 the disease was a serious problem.
Baily (1983) explains the occurrence of EFB as having the propensity, “…to remain inapparent, then to appear, sometimes in a very severe form, and then frequently to disappear spontaneously, especially after midsummer…”. Thompson and Brown (2001 see summary) indicate that yearly recurrence of the disease in infected apiaries is particularly problematic in the UK.
In Switzerland, incidences have increased in recent years (Forsgren et al. 2005). In that country, PCR techniques were used to detect European foulbrood in colonies with and without symptoms, (Belloy et al. 2007 see summary). It was found that in colonies without symptoms in apiaries where other colonies were symptomatic, 90% of the adult bees carried the bacteria. In apiaries without symptoms, but near symptomatic apiaries, 30% of the colonies carried the bacteria, and in apiaries far from symptomatic apiaries, the bacteria could not be detected. This means that it is possible that the bacteria may not be present in regionally isolated areas.
Cultural control
There are limited options for possible cultural control of this disease. However, as noted above, treatment may not always be necessary in all cases if conditions change that result in disappearance of the disease. Control is sometimes necessary though. Re-queening the colony may have some benefit, due to a break in the brood cycle, and supplying a queen that is more prolific (Shimanuki 1997). There is some evidence of genetic resistance towards the disease (McKee et al. 2004, Shimanuki 1997), but there are no known lines/breeds that are resistant to EFB, including lines bred for hygienic behavior (Spivak per comm). Hygienic lines are however clearly resistant to American foulbrood.
Due to the infectious activity of the bacteria on contaminated combs, moving combs and equipment should be expected to cause cross contamination. In some countries, destruction or sanitation of infected combs and equipment is required. As of 2008 in Switzerland, European foulbrood is a notifiable disease which requires sanitation of apiaries, without the use of antibiotics. This includes the process of burning every infected and weak colony. In a Swiss study, Roetschi et al. (2008 see summary) showed that this process was not very effective as 5 out of 8 sanitized apiaries were reinfected one year later. However, destroying contaminated equipment has proven effective in another study (see “Chemical control”).
Chemical control
In the US, as of this writing 2009, the antibiotic Oxytetracycline HCL soluble powder (OTC), trade name Terramycin is the only product labeled for the control of European foulbrood. Various concentrations are available. This means that users of this product should pay particular attention to the product label to deliver the correct dose, or contact their local state beekeeping inspector or extension specialist for assistance.
Terramycin is also registered for the use in controlling American foulbrood, although it has been established that American foulbrood is expressing resistance to this drug in the U.S. (Miyagi et al. 2000). An equivalent study on whether or not European foulbrood is expressing resistance to Terramycin in the U.S. could not be found. There is a study that investigated European foulbrood OTC resistance in the U.K. and it found that resistance was not occurring (Waite et al. 2003b see summary). However, use of Terramycin in the U.K. is greatly different then in the U.S., so this may not give any indication to its effectiveness in the U.S. Use of Terramycin as a precautionary, or prophylactic, method to prevent European foulbrood in non-symptomatic colonies, even in infected apiaries, is not recommended (Thompson and Brown 2001 see summary).
A promising method for controlling European foulbrood has been developed in the U.K., which involves the combination of removing contaminated equipment with the “shook swarm” method along with the use of antibiotics (Waite et al. 2003a see summary). As mentioned above, recurrence of the disease can be a major problem. This method seems to help control recurrence the following year, in addition to disease control the year implemented. See the research summary, Shook Swarm and OTC Antibiotics for European Foulbrood Control for more information. A trial of this method in the U.S. was not found.
Research Publication Summaries:
- Distribution of European Foulbrood in Apiaries With and Without Symptoms
- Infection of European Foulbrood Before and After Apiary Sanitation
– Antibiotic Treatment Study Summaries:
- Shook Swarm and OTC Antibiotics for European Foulbrood Control
- Assessment of Precautionary Antibiotic Treatments for European Foulbrood in Infected Apiaries
- Resistance Trial of European Foulbrood to OTC
References
- Baily, L. (1956). Aetiology of European foulbrood; a disease of larval honeybee. Nature 178: 1130.
- Baily, L. (1960). The epizootiology of European foulbrood of the larval honey bee, Apis mellifera Linnaeus. Journal of Insect Pathology 2: 67-83.
- Bailey, L. (1981). Honey Bee Pathology 124p.Academic Press Inc. (London) LTD.
- Bailey, L. (1983). Melissococcus pluton, the cause of European foulbrood of honey bes (Apis spp.). Journal of Applied Bacteriology 55: 65-69.
- Bailey, L. (1985). Melissococcus pluton and European foulbrood. Bee World 66: 134-136.
- Baily, L. and Collins, M. D. (1981). Reclassification of Streptococcus pluton (White) in a new genus Melissococcus, as Melissococcus pluton nom. rev.; comb. nov. Journal of Applied Microbiology 53(2): 215-217.
- Belloy, L., Imdorf, A., Fries, I., Forsgren, E., Berthoud, H., Kuhn, R., Charriere, J. (2007). Spatial distribution of Melissococcus plutonius in adult honey bees collected from apiaries and colonies with and without symptoms of European foulbrood. Apidologie 38: 136-140. Read summary here
- Delaplane, K. (1998). Strictly for the hobbyist: European foulbrood and its control. American Bee Journal 138(10): 736-737.
- Djordjevic, S. P., Noone, K., Smith, L, and Hornitzky, M. A. Z. (1998). Development of a hemi-nested PCR assay for the specific detection of Melissococcus pluton. Journal of Apicultural Research 37(3): 165-174.
- Forsgren, E., Lundhagen, A. C., Imdorf, A., and Fries, I. (2005). Distribution of Melissococcus plutonius in honeybee colonies with and without symptoms of European foulbrood. Microbial Ecology 50: 369-374.
- Herbert, E. W., Chitwood, D. J., and Shimanuki, H. (1987). Chalkbrood research at Beltsville. American Bee Journal 127: 488-491.
- Herbert, E. W. and Shimanuki (1984). An update on European foulbrood research in New Jersey. American Bee Journal 124(6): 472-473.
- Hornitzky, M. A. Z. and Smith, L. A. (1999). Sensitivity of Autralian Melissococcus pluton isolates to oxytetracycline hydrochloride. Australian Journal of Experimental Agriculture 39: 881-883.
- Kanbar, G., and Engels, W. (2003). Ultrastructure and bacterial infection of wounds in honey bee (Apis mellifera) pupae punctured by Varroa mites. Parasitology Research 90: 349-354.
- Lehnert, T., and Shimanuki (1980). European foulbrood disease control in honey bee colonies used for blueberry and cranberry pollination. American Bee Journal 120: 429-430.
- McKee, B. A., Goodman, R. D., and Hornitzcky, M. A. (2004). The transmittion of European foulbrood (Melissococcus plutonius) to artificially reared honey bee larvae (Apis mellifera). Journal of Apiclutural Research 43(3): 93-100.
- Miyagi, T., Peng, C. Y. S., Chuang, R. Y., Mussen, E. C., Spivak, M. S., and Doi, R. H. (2000). Verification of oxytetracycline-resistant American foulbrood pathogen Paenibacillus larvae in the United States. Journal of Invertebrate Pathology 75(1): 95-96.
- Morse, R., A. and Nowogrodzki, R. editors (1990). Honey Bee Pests, Predators, and Diseases 474p. Cornell University Press. Second edition.
- Pinnock, D. E. and Featherstone, N. E. (1984). Detection and quantification of Melissococcus Pluton infection in honeybee colonies by means of enzyme-linked immunosorbent assay. Journal of Apicultural Research 23(3): 168-170.
- Roetschi, A., Berthoud, H., Kuhn, R., Imdorf, A. (2008). Infection rate based on quantitative real-time PCR of Melissococcus plutonius, the causal agent of European foulbrood, in honeybee colonies before and after apiary sanitation. Apidologie 39: 362-371. Read summary here.
- Shimanuki, H. 1997 Bacteria, Chapter 3 from Morse, R., A. and Flottum, K. editors. Honey Bee Pests, Predators, and Diseases 718p. A. I. Root Company. Third edition.
- Shimanuki, H. and Knox, D. A (2000). Diagnosis of honey bee diseases. USDA-ARS Agriculture Handbook Number 690 : 61p.
- Shimanuki, H., Knox, D., and Feldlaufer, M. (1992). Honey bee disease interactions: The impact of chalkbrood on other honey bee brood diseases. American Bee Journal 132(11): 735-736.
- Smith, B. (2009). Lab Diagnosis of European Foulbrood. http://extension.org/pages/Lab_Diagnosis_of_European_Foulbrood
- Thompson, H. M. and Brown, M. A. (2001). Is contact colony treatment with antibiotics an effective control for Europan foulbrood? Bee World 82(3): 130-138. Read summary here.
- Tomkies, V, Flint, J., Johnson, G., Ruth, W., Wilkins, S., Danks, C., Watkins, M., Cuthbertson, G., Carpana, E., Marris, G., Budge, G., and Brown, M. (2009). Development and validation of a novel field test kit for European foulbrood. Apidologie 40: 63-72.
- Truper, H. G. and de Clari, L. (1998). Taxonomic note: erratum and correction of further specific epithets formed as substantives (nouns) ‘in apposition’. International Journal of Systematic Bacteriology 48: 615.
- Waite, R. J., Brown, M. A., Thompson, H. M., Bew, M. H. (2003a). Controlling European foulbrood with the shook swarm method and oxytetracycline in the UK. Apidologie 34: 569-575.Read summary here.
- Waite, R., Jackson, S., and Thompson, H. (2003b). Preliminary investigations into possible resistance to oxytetracycline in Melissococcus plutonius, a pathogen of honeybee larvae. Letters in Applied Microbiology 36: 20-24.Read summary here.
Page Authors: Michael Wilson and John Skinner, University of Tennessee. 2009.
Photographs by: Michael Wilson, except American foulbrood USDA photo noted above.
Acknowledgments: We appreciate the excellent review and editing provided by Dr. Dewey M. Caron, Affiliate Professor OSU and Dr. Jeff Harris, USDA.